

|
|
|
Three Things to Get Started |
||
|
1. Contact instructor |
2. Know the goals of course & expectations
|
3. Know your progress |
|
Send email to me to confirm that you are in the class. Ken Costello, Instructor chm107@chemistryland.com (480) 202-2993 Click on image of bird in cage on right to see the bird cage trick. |
Syllabus for CHM-107 as web page
|
Your scores will be shown in Sapling Learning. |
 |
||
|
Below are links to
your online textbook tutorials, quizzes, reading assignments, and exercises. |
|||
CHEMISTRY & SOCIETY: MAGICIAN & AUDIENCE by Ken Costello |
|||
| INTRODUCTION | |||
Pitfalls to Learning Pitfalls of Learning Chemistry Tutorial (2/1) NEW >> How to Create a Sapling Learning Account (2/3) |
 |
||
Chemistry in a New Light Chemistry
in a New Light Tutorial (2/6) |
 |
||
|
Scientific Skepticism:
The next two tutorials develop your skills at scientific skepticism and help you understand why societies make poor choices. |
|||
|
PROBLEM WITH AUDIENCES Audiences come to enjoy a performance. Their frame of mind is to be entertained. Not much interest is given to what happened in preparation of the performance or what happens after the performance. This makes them uninformed to the full depth of what the performance really should mean to them. As citizens and consumers we often have the same shallow insight as audiences. We are focused just on the product, results, performance, or solution. This makes us rather gullible and vulnerable. |
 |
||
|
WHAT HAPPENS BEHIND THE SCENES To gain the ability to see through tricks and to totally evaluate what is being presented, we need to step behind the scenes. This is true whether it's a card trick, a chemistry trick, or anything in between. A2: Read tutorial about What Happens Behind the Scenes. (2/15) Quiz on both "Problem with Audiences" and "What Happens Behind the Scenes" at Sapling Learning (2/18) |
 |
||
VIDEOS REAL OR FAKE? In this exercise you will try to determine if the 9 videos listed are fake or not. These are listed in Sapling Learning. First try to search the web or see if you find information on whether if they are fake or real. If you can't find out, then use your judgement to explain why you think the videos are real or fake. I'm more interested in what our observations or reasoning behind your decision rather than being accurate if it is real or not. In other words, this is practice on using critical thinking and scientific skepticism. (2/21) |
 |
||
|
Three Early Uses of Chemistry and Impact on Early Societies |
||
|
|
B1 : Stone Tool Technology (2/23) |
 |
 |
||
|
B3: Preservation of Food (2/25) Quiz on Early Chemistry (B1, B2, & B3) at Sapling Learning (2/27) |
 |
|
|
Building Blocks The world around us including ourselves are made from building blocks. (Reading assignments from textbook will be added soon to supplement the below tutorials) |
||
 |
C1: Building Block Intro (3/1) |
 |
|
C2: Protons, Electrons, and Neutrons Built from Energy (3/2) |
|
|
|
C3: Elements Built from Protons, Electrons, and Neutrons (3/3) |
|
|
|
|
||
|
|
||
|
C6: Building Blocks for Organic Compounds (3/6) Quiz on Building Block tutorial (C1, C2, C3, C4, C5, & C6) on Sapling Learning (3/7) |
|
|
Humans are normally uncomfortable with chaos and complexity. They want to simplify it. The development of the Periodic Table of the Elements tells that story. Calm the Chaos tutorial (3/9) Quiz at Sapling Learning (3/10) |
 |
|
CHEMISTRY AND SOCIETY IN THE NEWS |
||
|
Our Society had three problems which we solved using chemistry; however, in doing so, we created new problems with negative consequences. |
||
Society
Problem #1: We want a multitude
of products that are cheap and readily available. |
||
|
Society's Solution: Mass Production with global distribution of products. |
||
| Positive consequences: We are all materially very wealthy. Owning many more things than our ancestors ever dreamed of. Because of mass production of food products, we also have access to more food than our ancestors ever dreamed of. | ||
|
1st
Negative Consequence of Mass Production: Pollution
of Air, Water, and Soil (Reading assignments from textbook will be added to supplement the below tutorials) |
 |
|
 |
READ
INTRO (3/17) |
|
|
D1:
Misconceptions about Air
(3/19) |
 |
|
|
D2:
Composition of Clean Air and Polluted Air
(3/23) Using "Chemistry & Society in the News" link above, do assignment in Sapling Learning exercise titled "In the News: Record Air Pollution in Beijing" (3/26) |
|
|
|
D3: Solubility and Water's properties. How that contributes to contamination. |
|
|
|
2nd Negative Consequence of Mass Production:
Global Warming (Reading assignments from textbook will be added to supplement the below tutorials) |
 |
|
Tutorial #E1: Global Warming: Balancing Heat and Cold (4/3) Chemistry in Context: Chapter 3: The Chemistry of Global Climate Change. Read pages 106-116 & 120-125 Quiz on Global Warming now on Sapling Learning (4/5) |
||
| Exercise #E2 : Ice Cores & Global Warming: Finding data about carbon dioxide and methane on the Web (4/8) |
 |
|
|
3rd Consequence of Mass Production: Fuel
depletion, Greenhouse Gases, Pollution (Reading assignments from textbook will be added to supplement the below tutorials) |
 |
|
|
Tutorial #F1: What is Energy? Where do we get it? What are the consequences? (4/11) Quiz on Energy is now on Sapling Learning (4/12) |
||
|
4th Consequence: Unhealthy
Food |
||
Tutorial #G1: Shortened Life for Longer Storage Life (coming this Spring) |
||
|
Society Problem #2: Find raw materials
that allow easier and faster production of manufactured goods. (This
problem is related to problem 1) This material should be cheap, lightweight,
waterproof, inert, and moldable.
|
|
| Society's Solution: Polymers: Chaining together simple molecules allows the creation of a variety of materials known as polymers or plastics. These simple molecules primarily come from petroleum. |
 |
| Positive impact on society:
Plastics contribute to our material wealth because they are cheaper to manufacture. Cars are lighter in weight and therefore produce less pollution. Our clothes are warmer, last longer, and are easier to care for. Things are cheap enough to be disposed of; eliminating cleaning and use of water and soap. Plastics are waterproof and are durable in all weather conditions. Plastic containers or bags take up less space at home or in the landfill. Some plastics can replace body parts. |
 |
| Negative consequences:
• Because of plastics' durability, they do not decompose easily in landfills or as litter. • Some plastic holders for beverage bottles have strangled marine animals. • Plastics are made from petroleum which is a limited resource. |
 |
|
Tutorial
#H1: What are Polymers? How are they used? The consequences of using
polymers. (4/15) Quiz on Polymers is here. Send answers via email. Score will be recorded in Sapling Learning(4/18) Exercise H2 (extra credit): Visit http://H2No.org/PlasticCodes.asp and Find plastic items around the house that has these codes and report what polymer code is printed on them. Give at least three (If above link doesn't work, do a Google search for "plastic codes".) Send answers via email. (12/1) |
 |
|
Society Problem #3:
For refrigeration, we need a gas that upon compression will turn to
liquid at room temperatures. It also cannot be flammable and should
be cheap to make. |
|
| Society's Solution: Combine a few chlorine atoms, fluorine atoms, and carbon
atoms to make a variety of compounds called chlorofluorocarbons. |
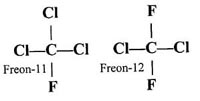 |
| Positive Impact on Society: The comfort of refrigeration was enjoyed in homes, cars, businesses, and elsewhere. This gas was also used to make refrigerators and freezers. Chlorofluorocarbons were useful in spray cans, the making of Styrofoam and other foam-like plastics, fire extinguishers, cleaning solvents, and other products. It had a huge positive impact on society, which is why accepting its negative consequences was difficult. |
 |
| Negative consequence: The chlorofluorocarbons worked their way into the upper stratosphere and destroyed ozone, which blocks the sun's high energy ultraviolet light. Excess ultraviolet light damages skin, breaks DNA, causes skin cancer, damages eyes, kills plants, and causes many more problems. Life on Earth will cease to exist if too much UV light gets through the atmosphere. A hole in the ozone layer around the south pole has grown to 10 million square miles (twice the size of United Sates). People in Antarctica and southern tip of South America are now warned to wear thicker clothing for protection. The ozone level over the whole Earth has also dropped. |
 |
|
"Protecting the Ozone Layer" |
|
|
Tutorial #I1: What is the ozone layer?
How is ozone destroyed? Why is ultraviolet light dangerous? (coming
this Spring) Quiz on Protecting the Ozone Layer (coming this Spring) |
 |
FINAL EXAM Link to Online Final Exam (Everyone takes this one. This is not in Sapling Learning. You send your answers via email to CHM107@chemistryland.com) |
|