
The average temperature of the Earth is 15°C (59°F). This is cool but not cold. It's the temperature of a nice spring or fall day. It keeps this average temperature because there's a balance of heat gain from the Sun (yellow lines), heat being lost to the freezing cold of outer space (blue lines), and heat being returned to the Earth by the atmosphere (orange lines). If the Sun's heat is blocked, the Earth can freeze quickly. If something keeps in the heat too much, Earth can also heat up quickly, too.
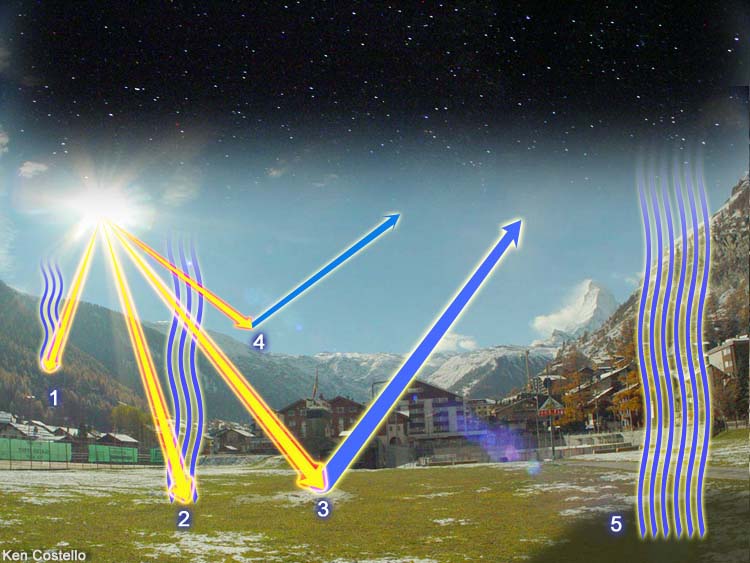
Energy from the sun follows different paths. Starting with #1 ray of light, we see it entering the atmosphere and being absorbed by the air. Some of this heat radiates back to space. The #2 ray of sunlight hits the ground and heats the ground. Some of this heat radiates back up to space. The #3 ray reflects off the ground and goes back into space. The #4 ray gets reflected back to space by the atmosphere. Even where the sun isn't shining, heat is radiating out to space. In other words, a lot of energy hits the Earth but the Earth either reflects the heat or radiates heat back into space. Without some way of returning this heat going up into space, the Earth would be a frozen wasteland. Fortunately, we have some gases in the air which intercept this energy and radiate much of it back to the ground.


Carbon Dioxide: Carbon dioxide is the building block for all plant growth. However, too much in the atmosphere is believed to be the second leading cause of global warming (second to water vapor). Looking at samples of ice dating back 160,000 years gives us some clues that CO2 and world temperature are connected. Samples of ice are taken with a core drill (see below)

Left photo: Mark Twickler, University of New Hampshire. Top photo: Lonnie Thompson,
Ohio State University. Bottom right photo: Kendrick Taylor, Desert Research Institute.
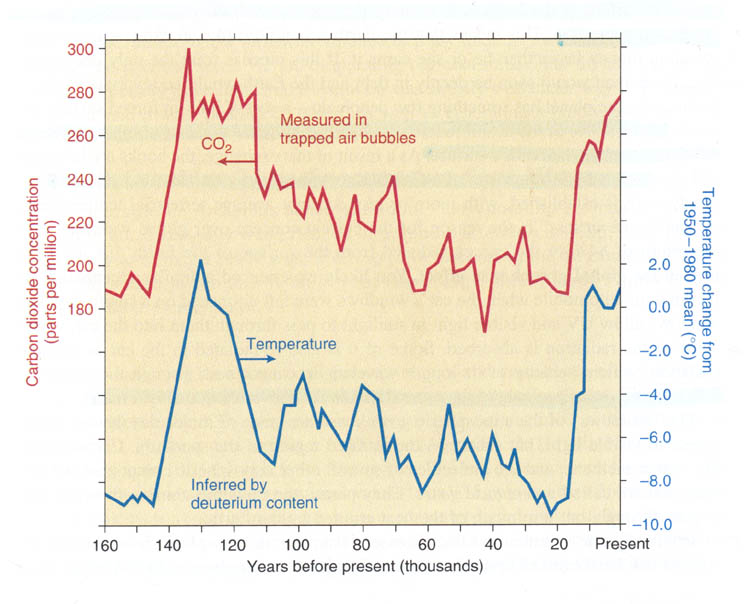
This graph shows that when CO2 levels were high the calculated temperature was also high. It appears that 130,000 years ago there was a high level of CO2 and the temperature was higher as well (Why it was high I'm not sure). In the last 15,000 years it appears we are on an upswing again. So global warming might be a natural cyclic process. Unfortunately, since we are on the upswing, adding more CO2 in the atmosphere may be the wrong time to add more heat. If we were to produce more CO2 30,000 years ago, it may have helped us get out of the ice age.
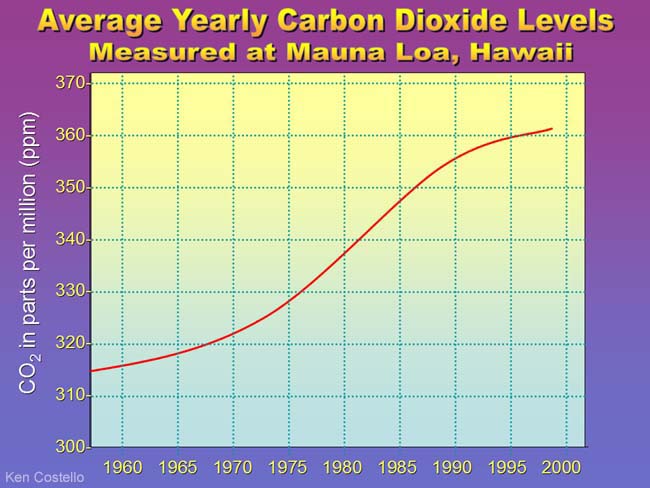
Notice the temperature difference from 20,000 years ago to present is a difference of about 10°C or 18F. That's the difference between a day where water is always frozen (32°F) to a cool 50°F long sleeve shirt day. Or you can look at it as a hot Phoenix summer day of 118°F turning into 140°F where all plants die, all cars overheat, and air conditioners can't cope. So 18°F (10°C) makes a big difference in the world. More alarming is that in the above graph we can find jumps of 50 parts per million of CO2 but it took at least 10,000 years. We've had a jump of almost 50 parts per million in the last 40 years. See the graph below.
Carbon Cycle

This graphic illustrates what happens to carbon including carbon dioxide. The "bmt" units stand for billion metric tons. A metric ton is 2,200 lbs., about the weight of a compact car. One billion metric tons would be the weight of a billion (1,000,000,000) compact cars.
Plants
absorb 110 billion metric tons as they grow. In photosynthesis CO2
is turned to sugar molecules. Here's the simplified reaction:
6H2O + 6CO2 + light -> C6H12O6
(glucose) + 6O2
Glucose is utilized as a building block for the rest of the plant.
Animals on land (including us) place about 55,000,000,000
metric tons of CO2 in the air. Each person's own breath contributes
about 1,000 lbs or CO2 to the atmosphere each year. Our burning of food calories is basically
the reverse reaction of photosynthesis shown on he left:
C6H12O6 (glucose)
+ 6O2 -> 6H2O + 6CO2
+ Energy


The burning of fossil fuel (petroleum) for energy in factories and automobiles and for heating homes and businesses contribute 23 billion metric tons. If you drive 12,000 miles per year and your car gets 20 miles per gallon, your car contributes 12,000 lbs (5 metric tons) of CO2 per year (1 gallon gasoline yeilds 20 lbs CO2).

Photo: USDA Forest Service
