|
|
Before you can understand air pollution, you need to have
an understanding of clean air. |
|
Composition
of Clean Air |
|
|
|
In the fall of 2002 I got to go to Switzerland.
On the morning of November 6th, I had to leave the village of Zermatt,
but wanted to take one more look at the Matterhorn. The air was absolutely
clear. Cleaner than I have ever seen air anywhere. The Matterhorn jumped
out at you. By the way, Zermatt does not allow automobiles. People walk
or ride electric vehicles. The train that goes there and the trolley that
takes you to the ski lifts also run off of electricity. |
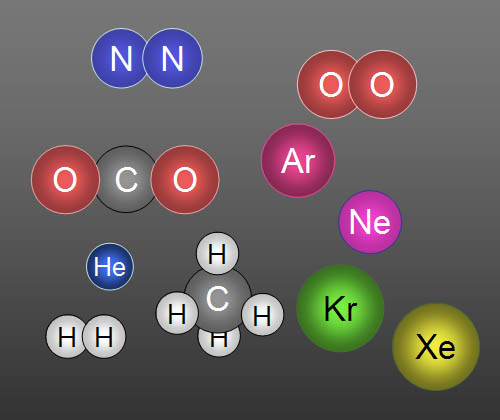 |
Here are 10 gases that make up clean air: In
order of highest to lowest concentration they are Nitrogen, Oxygen, Argon,
Carbon dioxide, Neon, Helium, Methane (CH4), Krypton,
Hydrogen, and Xenon. Five of them travel alone, so we call them atoms.
For example, a helium balloon contains atoms
of helium (He), but an oxygen tank contains molecules
of oxygen (O2). When there's two or more atoms are
bonded together, they are called molecules. |
|
|
NITROGEN
(N2) #1:
8 out of 10 atoms
(or molecules) of air are made of nitrogen. Nitrogen gas can't be seen
but when it is cooled to -320 °F (-195°C) it turns to a liquid,
which you can see. Being this cold makes it handy for many things. Shown
here, it is being used to quickly freeze cream and sugar to make ice cream. |

|
NITROGEN #2:
Liquid nitrogen is also used to cool certain metals to the point where they
become superconductors. Superconductors conduct electricity (electrons)
without any resistance. In this picture a rectangular shaped magnet had
been dropped onto a metal disk that had been cooled enough so that it was a superconductor. As the magnet fell toward the metal disk,
the magnetism in the magnet caused currents of electricity to flow inside
the metal disk. The currents created in the metal disk by the falling magnet
made that part of the metal disk a magnet itself. The falling magnet then
feels the repulsion of the magnet it just created. The falling magnet will
then stop just above the metal disk. Electricity continues to flow in the
metal disk constantly creating a mirror image magnet that repels the real
magnet. This will continue forever as long as the metal is cool enough to
stay a superconductor. |
|
|
NITROGEN #3: Oxygen reacts
with many things including paint. Therefore, expensive works of art and
other rare relics are stored in pure nitrogen. Nitrogen gas is fairly inert
and doesn't react (combine) with most other substances, so it is used
to protect items and keep oxygen away.. |
Bad Nitrogen
 |
|
NITROGEN goes bad: Under
high temperatures, like in a jet engine or car engine, nitrogen will combine
with oxygen to form a class of toxic
compounds called nitrogen oxides. The simplest having one nitrogen and
one oxygen (NO). Others have two nitrogens and one oxygen (N2O),
one nitrogen and two oxygens (NO2), and the fourth
has two of each (N2O2). Their
names (in order) are nitrogen oxide, dinitrogen oxide, nitrogen dioxide,
and dinitrogen dioxide. Nitrogen dioxide has a brownish appearance and
is often what you see in polluted cities. |
 |
Oxygen
#1: 2 out of 10 atoms/molecules of air
is oxygen. Oxygen is produced by plants and is consumed by animals. Animals
need oxygen to "burn" the food they eat in order to get the calories
(energy) they need. The way animals use oxygen to burn food is different
than a fire, but it produces the same products of carbon dioxide and water.
Of all of the gases that make up air, oxygen is the most reactive. |
Oxygen is Everywhere in This Photo
 |
|
OXYGEN #2: Oxygen
is everywhere. As a gas it makes up 20% of the air. Some oxygen gas is
dissolved in water, and some oxygen gas is in the pores in the ground.
However, there's a lot more oxygen in this picture that we don't
normally consider. 90% of the weight of the water in the river is oxygen
atoms. Remember water is H2O. The one oxygen in H2O
weighs 8 times has much as the two hydrogen atoms combined. The
trees and grass are made of carbon, oxygen, hydrogen, and nitrogen, with
oxygen accounting for about half of a plant's weight. The rocks,
the soil, and the mountains contain metals and silicon that is bound to
oxygen, which makes up about 1/2 of their weight. Think about it; one
half of a mountain's weight is from oxygen. The only thing in the picture
that doesn't contain oxygen are the metal rails. |
|
|
| OXYGEN #3:
In the movie Total Recall, there was an ancient alien factory that could
release the oxygen from the Martian soil. This is actually possible. Extreme
heat will cause the oxygen to separate from the metals and non-metals that
it was bound to. Most of Martian soil is iron oxide, which would turn to
iron metal and oxygen gas at high temperatures. In the pictures above, Arnold
presses the touch sensitive on "button" of the oxygen generator.
Next he gets blown out of the factory into the thin Martian air, which
causes the air in his body to expand. Fortunately, the oxygen rushes out
of the top of the mountain that housed the factory and they show Mars getting
an atmosphere similar to Earth's. Actually, this atmosphere would be pure
oxygen, which is not good to breath for long periods and would present extreme
fire hazards. It's lacking the 80% nitrogen. |
|
|
ARGON:
Argon is 1 out of 100 atoms/molecules of air. Argon is an inert gas that
is used to pressurize light bulbs. Being inert it doesn't react with the
tungsten metal, which makes the filament. The argon gas also helps keeps
the tungsten metal atoms on the surface of the filament from jumping off
the filament. Argon gas is also used in welding to push away the air so that the oxygen in the air won't react (oxidize) the metal being welded. |
|
|
CARBON DIOXIDE:
Carbon dioxide only makes 3 out of 10,000 atoms/molecules of air; however,
it supplies the carbon that plants use to make leaves, trunks, roots, etc.
It's hard to believe that the weight of giant redwood trees came from the
small amount of carbon dioxide gas in the air. |
 |
NEON:
2 out 100,000 atoms/molecules of air. Neon is a clear inert gas; however,
high voltage can strip electrons off neon atoms. As the electrons fall back
into place, they give off ultraviolet light (black light), which you can't
see. However, the glass tubes are coated with different phosphor powders,
which glow when ultraviolet light hits them. |
|
|
HELIUM:
5 out of 1,000,000 atoms or molecules of air are helium. Helium is the second
lightest gas (hydrogen is lightest). It is used to fill toy balloons and
weather balloons. It is so inert that there is no danger of igniting nor
will it combine with other elements. For this reason it is used in welding
by having helium gas surround an electric arc that is melting a metal. The
flow of helium gas keeps the oxygen away from the metal so that the oxygen
won't oxidize (rust) the metals being welded. |
|
|
| METHANE:
2 out of 1,000,000 atoms/molecules of air are methane molecules. Bacteria
is a big source of methane gas and this type of bacteria is found in termites
(see huge termite mound on the left), cattle (cow flatulence), rice paddies, swamps
(called swamp or marsh gas), and in the sea bed. In the sea bed, methane
can be found trapped in ice (see person holding ice that's on fire). Methane
also comes from petroleum fields and is the natural gas you cook with. An
embarrassing source of methane is from the eating of beans. |
 |
KRYPTON:
1 out of 1,000,000 atoms/molecules of air is krypton gas. You may remember
krypton as the name of the planet that Superman came from. The pieces of
the planet that fell as meteorites were called kryptonite. The element krypton is used in lamps in
a similar way that argon is used (see above). The word krypton comes from
"Krypto" meaning hidden. The gas krypton was in a way hidden because
it took quite a while for scientists to discover it. |
 |
HYDROGEN:
1 out of 2,000,000 atoms/molecules of air is hydrogen. Hydrogen is the lightest
element, which is why is was the preferred gas to fill blimps and dirigibles.
Unfortunately, hydrogen is very flammable, which led to the catastrophic
tragedy of Germany's Hindenberg airship. |
|
|
XENON:
87 atoms out of 1,000,000,000 (one billion) atoms/molecules of air are xenon.
Like argon and krypton, xenon is used as the gas in lamps. This one is for
a car's headlamp. These are probably the ones that look more blue. |
|
|
| WATER VAPOR:
The other gas we didn't list before is water vapor. The concentration of
the other gases are pretty consistent, but water vapor varies greatly. Using
a fish-eye lens, I took this picture of a double rainbow in Geneva, Switzerland.
It had rained all day, but just before the sun went down, the clouds parted
enough to allow the sunshine to strike the droplets of liquid water still
in the air. Water in vapor form (gas) is not visible. |
 |
One Last Look
at Composition of Air: Imagine the volume of
air in a typical classroom that is 30 feet by 30 feet with a 10 foot high
ceiling. Also assume, we separated all the gases. Oxygen would cover the
room to about 2 feet deep. Nitrogen would fill almost to the ceiling (another
8 feet minus a couple of inches). Argon gas would fill a one inch layer
over the whole room. The remaining gases fill the last one inch. Carbon
dioxide has about the same volume of one student. Neon is 1.5 gallons. Helium
would fill a one liter bottle. Methane gas would fill someone's 1/2 liter
bottle. Krypton would fill a 12 oz soda can. Hydrogen would fill about half
of a 12 oz soda can. And xenon gas would have the volume of a pencil's eraser.
|
Composition
of Polluted Air |
Particulates
Part of the below tutorial is from Lab 1 (Air We Breathe) |
 |
The image on the left is from one of the dust storms that hit Mesa and Phoenix. We all know that breathing that dust will be bad for us. Even on days without a dust storm, there are plenty of particulates in the air which aren't healthy.
Surprisingly, it's not just what the particulates are made of, but the size of the particulates that also affects its effect on the lungs.
Note: In this context, "particulates" mean solid particles that are suspended in the air.
|
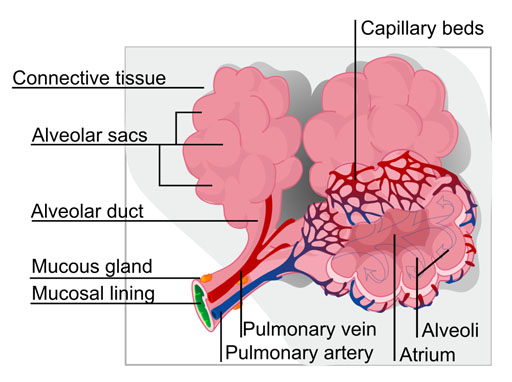 |
The lungs contain thousands of sacs called alveoli. This is where oxygen passes through the lining of the alveoli and enters the blood. This is also where carbon dioxide leaves the blood. Our nose hairs and mucous in the nasal passages and the bronchial tubes try to block particles that we breath in. However, if the particles are too small, they get by these defenses and get to the alveoli. If the particles are even smaller, they pass through the lining of the alveoli and even get into the blood stream. |
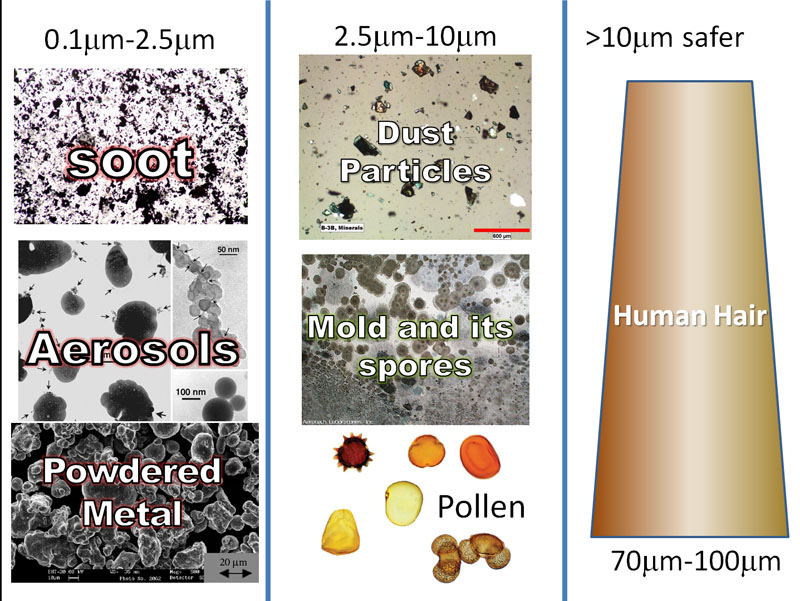
| (See image below) The human hair is about 70 to 100 millionths of a meter in diameter. A millionth (micro) of a meter is called a micrometer or micron for short. The symbol for a millionth (micro) is µ. So the human hair is 70µm to 100µm in diameter. Particles over 10µm are usually trapped by nose hairs and mucous in the nasal passages. So their danger is less. However, particles between 10µm and 2µm get past those defenses and get deep into the lungs. These are such things as dust particles, mold, mold spores, and pollen. They can irritate the lungs and cause severe reactions. Smaller particles (0.1µm to 2.5µm) are small enough to pass through the lung lining and enter the blood stream and reach all organs of the body. As you might guess, this can cause even greater health issues. These smaller particles can be soot (from combustion of wood, gasoline, diesel, etc.), aerosols (from sulfuric acid forming in the atmosphere and reacting with other particles), and finely powdered metal (from car brake pads, metal sanding, etc.). |
|
|
The air inside and outside our home contains numerous
particulates (particles suspended in the air). Below are some, plus their
size in microns. Anything under 10
microns is not visible to the eye and is more hazardous. By the way, dust mites live in your
bedding and pillows. They eat the flakes of skin that you shed.
Human Hair .................(70 - 100 microns)
Pet Dander ..................(0.5 - 100 microns)
Pollen ...........................(5 - 100 microns)
Spores from Plants ....... (6 - 100 microns)
Mold........................... (2 - 20 microns)
Smoke ........................ (.01 - 1 micron)
Dust Mite Debris ........ (0.5 - 50 microns)
Household Dust .......... (.05 - 100 microns)
Skin Flakes ...................(0.4 - 10 microns)
Bacteria....................... (0.35 - 10 microns)
As you can see, most of these dip down into the unhealthiest range of under 2.5µm.
|
|
As a defense, filters are employed to trap these particles. You may have heard of HEPA filters for vacuum cleaners
and room air filters. HEPA stands for "High Efficiency Particulate Arresting". Arresting meaning stopping.
These filters were first used to capture radioactive particles in the
air at the facility where the first atomic bomb was being built.
HEPA filters will only miss 3 out 10,000 particles 0.3
microns in size. For larger size particles, it misses even fewer of them.
It uses a mesh of fibers to accomplish this. Fibers can
be made from plastic, glass, or cellulose (like cotton fibers).
|
|
The fibers actually have openings much larger than the
particles that it can capture. So it doesn't act like a sieve that can
only capture particles larger than the sieve openings. As particles move
through the fiber mesh, they get wedged in the narrow spaces where the
fibers cross each other. The faster moving air actually helps particles
get wedged in between fibers. That's probably why it is called High
Efficiency Particulate Arresting. Of course, particles larger
than the openings will get caught between the fibers.
They call these mechanical filters because the particulates
are mechanically held (blocked or wedged) in the filter. |
|
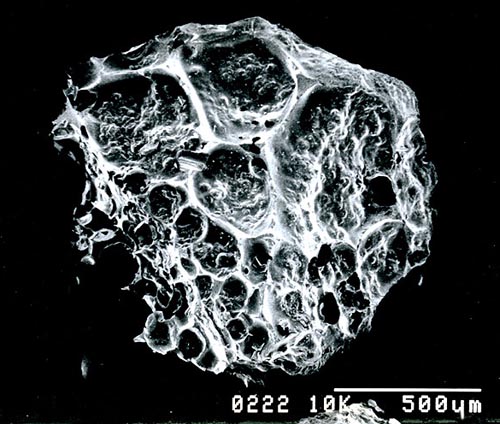
This is a granule of activated charcoal. The pits seen
are about 100 microns in size; however, these are the large pits. There
are others thousands of times smaller. So activated charcoal can act as
a mechanical filter by blocking particles larger than its smallest pores.
However, it can also trap certain substances that are so small that they can pass right through the
pores. Activated charcoal does this because certain substance are attracted to the surface
of the carbon. In other words, the carbon in activated charcoal acts like
fly paper to certain substances. Vapors from solvents like gasoline or
paint thinner stick to the surfaces and are trapped.
They call this adsorption (spelled with a "d").
Absorption (spelled with a "b") is when a thing soaks into something.
Adsorption
is when it sticks to its surface. |
|
This is the famous brown air of Phoenix. It's
appearance is from a mixture of dust, smoke, other particulates, and nitrogen
dioxide (which is brown). |
 |
Smoke from wood fires:
The smoke from a campfire or from a forest fire is quite dangerous partially because of the abundance of very small particles that are in the smoke. Most particles in wood smoke are smaller than the 2.5 microns (2.5µm). These particles don't normally get coughed up. They are so small that they get stuck into the lining of the alveolus sacs.
Perhaps the most dangerous component of wood smoke is the countless airborne particles (particulates). Airborne particles smaller than 2.5 microns are harmful because they are tiny enough to lodge inside lung tissue, while particles larger than 2.5 microns are coughed out. Researchers have discovered that the particles found in wood smoke are almost all smaller than 2.5 microns. |
| |
The Environmental Protection Agency lists the below health dangers for particulates:
- Irritation of the airways, coughing, and difficulty breathing
- Reduced lung function
- Aggravated asthma
- Chronic bronchitis
- Irregular heartbeat
- Nonfatal heart attacks
- Some cancers
|
|
One place to find particulates in the air is to look for what particulates have settled out of the air and onto our cars. In the dry climate in Arizona, particulates are not washed out of the air from rain, so cars get dusty pretty quickly. Also, the car seems to accumulate more road grime and dust when it's driven on the highways and streets. A few cars filter the air coming through the vents, but that still doesn't help when you roll down the windows. Again, most cars don't have a car cabin air filter. |
| SAMPLE OF OUTDOOR PARTICLES: Below is a sample taken from a dusty car using clear tape. What is mostly seen are lot of small particles of clear silica (sand/quartz). The photo doesn't show how clear these little particles appear. Also, the few cylindrical particles present look like pieces of plant material. When there was white paper behind the tape, many small black particles were seen. They, again, are likely soot particles and even particles from tires. So outside seems to have more mineral dust, more soot, and tire particles. |
|
|
POLLEN: Pollen is one of the common particulates in the air. For people with allergies, pollen is one of the biggest triggers of allergic reactions.
Pollen is stored on the anthers, which sit on the ends of stalks called filaments.
|
 |
The yellowish white spheres are pollen. The tiny white specks that you see in the photo are just adhesive from the tape that was used to capture the pollen.
|
|
DUST from SOIL: Fine particles in dirt are often blown into the air by wind and vehicles. Dust from soil is one of the common particulates in the air, especially in Phoenix and the surrounding area. |
|
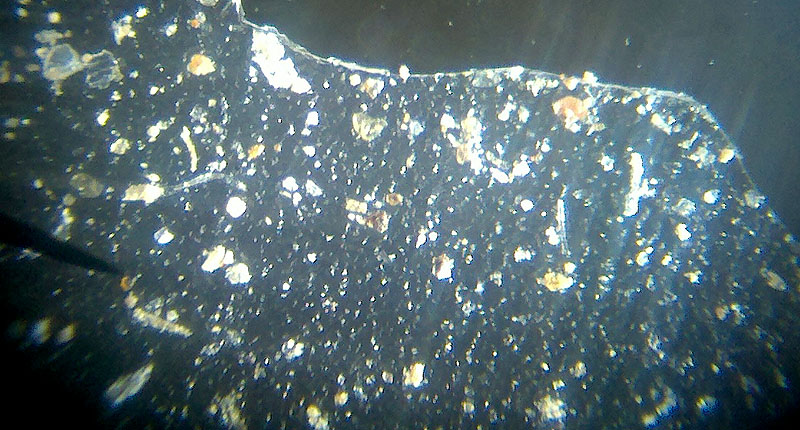
The soil sample collected shows a variety of minerals. Many are too large to get airborne, but the smaller ones can become dust pollution. Soil is mostly quartz with hundreds of other minerals. The darker grains are likely iron oxides or silicon dioxide (quartz) with iron or magnesium impurities. Soil will also have some pieces of plant material. None is that noticeable in this photo. Some soils are rich in organic (plant) materials, but Arizona soil doesn't have that much plant material in its soil. |
|
Here is the same sample without the white paper background. You can see that it changes the look of the particles. The lighter particles stand out more. The grains of minerals have a rough look. They also look clear, white, black, or may have some color in it. If it is a bright color, then it is probably not a mineral, perhaps a paint chip or piece of some insect. |
|
SOOT and SMOKE: The other particles that are often seen in air samples are carbon particles (soot). These come from unburnt gasoline and unburnt diesel. Fireplaces also put out a lot of soot, but fortunately in Phoenix fireplaces aren't used that much. Forest fires also put out a tremendous amount of ash and soot. So it's just not the fire that is dangerous about a forest fire; it's the smoke.
When we think of diesel smoke, we usually think of the large semi trucks. However, there are thousands of pickups that have diesel engines just in any one city. The small trucks also put out diesel smoke. Not as much as the large trucks nor as much as the truck in this photo. Rather sadly, some diesel truck owners actually program their truck's microcontroller to produce these large amounts of smoke when they want to. |
|
Nearly every school bus runs on diesel also. If the engines are cold, old, or not properly tuned, then a lot of diesel smoke gets breathed by school children around that bus. |
 |
The ultimate air pollution happens when a person smokes. The smoke is deliberately inhaled deep into the lung. The very small particles of smoke penetrate into the lung tissue.
This is a section of lung tissue from a smoker. You can see how the carbon particles get lodged around the alveoli. |
|
Gasoline engines also put out soot especially if the gasoline to air mixture is rich (too much gasoline). The inside of a tailpipe of a car is a good place to find carbon particles (soot). |
|
Here is the sample from the tail pipe.
Soot formed from unburnt gasoline or diesel are very small particles. They look like tiny black or gray specks under the microscope. In extreme magnification, they look like spheres. The reddish color in this image is probably from rust particles (iron oxides) coming from metal in the exhaust system. As mentioned before, the small size of these smoke particulate make them more dangerous because they can lodge in the alveoli of the lungs. |
|
Some ash particles from cigarettes or burning wood can be larger than the typical soot particles. They are also black or dark gray. Some have a bluish tint. These ashes in the photo were caught in a wad of cotton using an air pump. The strands are the cotton fibers.
Again, the larger particles are normally caught by the nose or sinus. Unfortunately, smoking means the smoke goes directly into the mouth where there is little filtering. So it gets into the lungs without much stopping it. |
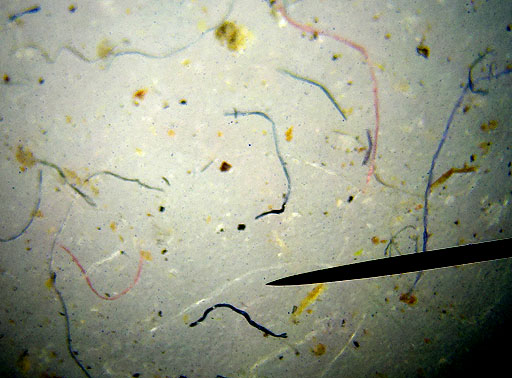 |
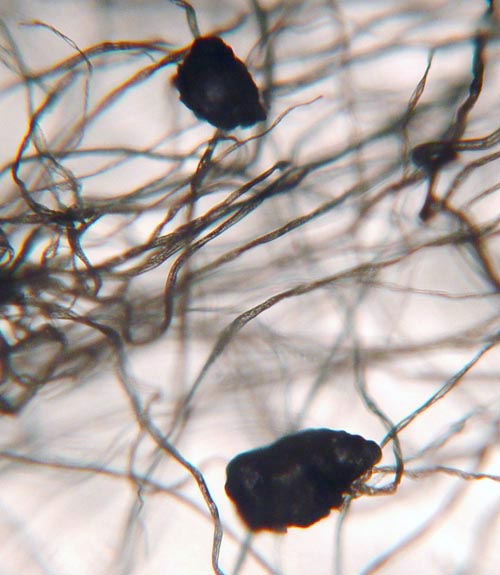
FIBERS:
The air we breathe also has a lot of fibers and hairs in it. These short strands of fiber are from such things as clothes, rags, carpet, and paper towels. The hairs may be from pets or people, and even insects. |
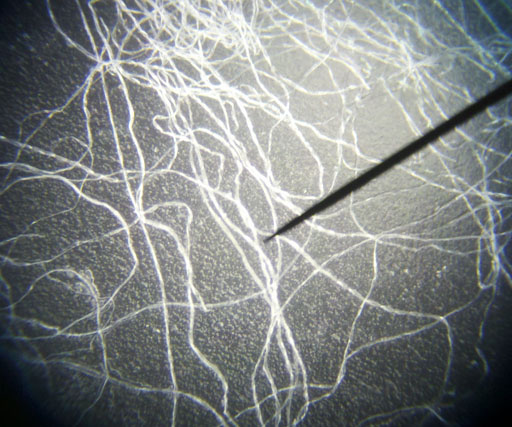 |
Cotton fibers look like flat ribbons that got twisted. |
|
These are cotton fibers at 250x magnification. |
|
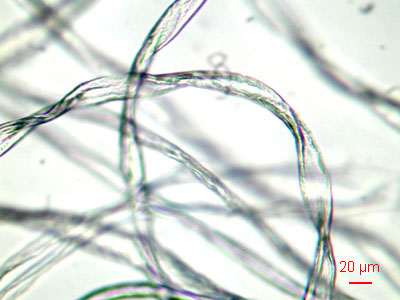
Synthetic fibers like nylon, polyester, and others are more round than cotton fibers. This magnification is about 100x.
Long fibers don't get in the air easily. It's the short ones that we often breathe. |
 |
This photo was shown above. The fibers that are more bent and twisted are likely cotton fibers. These have color because they are dyed cotton. A few fibers are less bent. Those are likely synthetic fibers. |
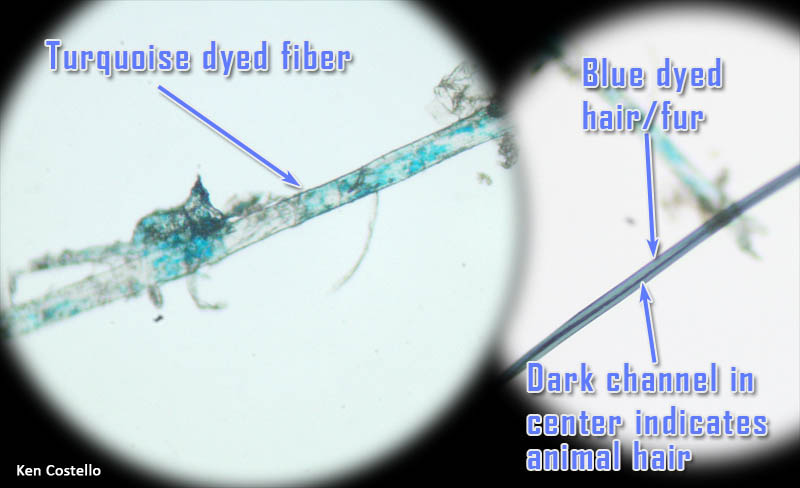
On the left is a synthetic fiber at about 250x magnification. Notice is it is not flat and twisted like cotton fiber. You can see some turquoise dye on (or in) the fiber. On the right is a strand of human or animal hair. Human and animal hair have a channel that runs through the middle of it. In high magnifications, scales on the outside of the hair can be seen.
|
|
DANDER AND DANDRUFF:
Both dander and dandruff are pieces of dead skin. Dander normally refers to the dead skin that comes off of animals like pet dogs and cats. Dandruff is dead skin from the scalp. Dandruff is usually larger and flatter than dander. |
|
If you are interested in more information about microscopy, you can visit the website developed by the National High Magnetic Field Laboratory at the Florida State University.
http://micro.magnet.fsu.edu/ |
Ozone
Part of the below tutorial is from Lab 3 (Air We Breathe) |
|
Ozone: The
Good, the Bad, and
the Useful
In the open fish market below,
ozone is affecting these students in three ways.
Good) Ozone 20 miles above them is helping to protect
their eyes and skin from UV radiation.
Bad) Ozone at the ground level from automobiles and some electrical devices is harming their
lungs.
Useful) Ozone generators used in the nearby businesses
kills mold and odors.
Useful) Ozone used by the city's water
treatment plant kills bacteria and microbes in the water they drink. |
|

|
|
This is the equation normally shown that
identifies the main contributors to ground
ozone. Car engines produce nitrogen
oxides (NOx). Unburnt gasoline supplies much of the volatile
organic compounds (VOC's). These combined with the energy from
the sun produce unhealthy levels of ozone.
This source of ozone pollution gets plenty
of media coverage. However, there are other sources of ozone we don't
hear much about... |
|
POISON IS GOOD?
You will often learn that chemicals which
are deadly are also in widespread use. One reason is that we need chemicals
to kill things we do not want around.
That is why the symbol for poison is smiling.
Poisons realize that even though we don't want to poison ourselves, we
do want them around to poison things we don't want. Ozone
is one of those poisons. |
|
|
For example, there is a lot in water that we do not want.
Ozone, acting as a poison, is often used to kill bacteria and other microbes
in water treatment plants.
Here is a ozone generator that puts out 35 lbs of ozone
per day. It is used in factories and municipalities for water treatment. |
|
|
There are even home versions
of these ozone generators. The larger one is for the whole house, the smaller
for under the sink applications. |
|
There are even small (1 foot cube) ozone washing machines to sterilize foods or dishes. |
|
Another model is for washing
hands and face with ozone sterilized water. |
|
|
Ozone can also be used to kill mildew, like we
see in bathrooms. Of course we usually use bleach to kill mildew. It's easier to buy,
but bleach does not easily decompose.
To kill mildew, ozone has to be in high concentrations,
too high for people to be in the room. Fortunately, ozone will revert
back to oxygen in a few hours and not be a continued threat. |
|
High powered ozone generators
are available for home use that can kill mildew. Like I said, it is not
safe to breathe, so you have to vacate the home while it is in high level
ozone generation mode. The units can then be adjusted to emit safe levels
of ozone for combating odors even while you are in the home. |
|
Restaurants and bars are
just a few places that may use ozone generators to combat odors, or if set
to "high," to disinfect food, utensils, or work areas. |
|
Motels, hotels, and hospitals
also use ozone generators to kill odors, reduce cigarette smoke, and kill
microbes. |
|
|
All of the above applications are good uses
of ozone. The thing to worry about is leakage from the high volume ozone
generators or exposure to excessive or extended levels of ozone from any
ozone generator. |
|
Another source of ozone are home air purifiers (air filters) that use electrostatic filters. These type of filters place a high voltage on a filter. This electrical charge will attract many particles in the air. It's a good approach except these devices produce some ozone. The amount of ozone is usually just under the EPA limit for ozone. Unfortunately, some people will have two or more of these kinds of filters in the same room or near each other. Then the level of ozone exceeds safe levels. |
|
|
Fortunately, there are detectors for ozone
to warn individuals of high ozone exposure. The more expensive ones are
electronic. They give a reading in a minute or so and are more accurate.
The others use chemicals embedded in paper that change color as they are
exposed to ozone. These take at least one hour to get a reading. Some require
a full 8 hours. |
Carbon Monoxide |
|
Carbon monoxide is another common pollutant. It's considered more of a hazard than ozone inside of buildings, which is why we see these in almost every home and hotel room. Like ozone, there are detectors for carbon monoxide (CO) as well. The one on the left is the common one we see mounted on the ceilings. The right one is a portable carbon monoxide detector. The bottom one is a color dot that goes dark in about 5 to 30 minutes if carbon monoxide is present. The speed depends on the concentration. The one shown is marketed to pilots of small aircraft to check to see if there is carbon monoxide in the cabin. These can be also placed into trucks and cars. |
 |
Carbon monoxide is called the silent killer because it odorless, colorless, and most people cannot sense that they are being poisoned with carbon monoxide.
It originates from a flame (combustion) where there is insufficient oxygen available to turn the fuel completely into carbon dioxide (CO2); instead carbon monoxide (CO) forms. Common sources are stoves, furnaces, automobiles, gas generators, camp fires, charcoal barbeque grills, and more. |
|
As the poster implies. A person may think they are suffering from too much alcohol, which in fact it could be carbon monoxide poisoning. Also, people think they may be suffering from the flu instead of realizing it as carbon monoxide poisoning. |
|
Carbon monoxide deaths are always in the news. After Hurricane Sandy hit New Jersey and New York city in October 2012, electricity went out. People turned to generators and charcoal grills for heat, but carbon monoxide killed many. That's why you see this public health notice that says to keep generators and charcoal grills out of and away from the house. |
| In a heavy snow storm in Boston in February of 2013, electricity went out and some people went to their cars and started the engine to stay warm. However, the depth of the snow blocked some of the exhaust and let carbon monoxide back into the car. Two people died. A teenage boy died in the car shown below. If you ever have to resort to staying warm in an idling car, be sure to shovel snow away from the exhaust system. Also be on the look out for symptoms of carbon monoxide poisoning (headache, nausea, dizziness, sleepiness, weakness, even diarrhea). |
|
|
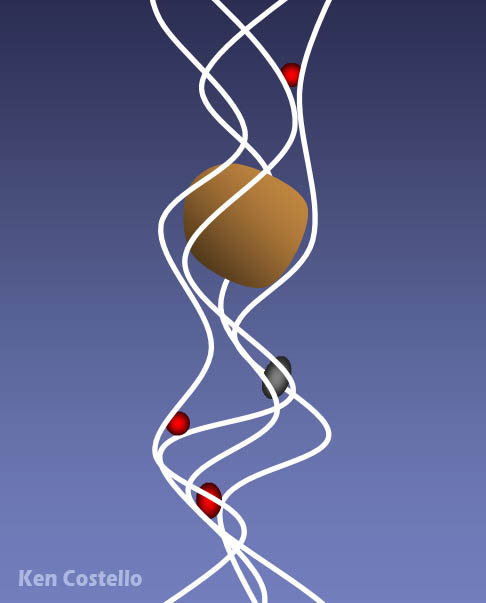
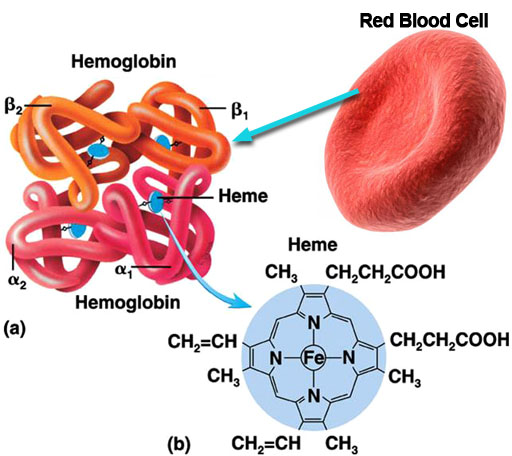
As you know, red blood cells carry oxygen from the lungs to the body and carries waste carbon dioxide from the body tissues to the lungs. The red blood cell is covered with millions of hemoglobin proteins. The tubes you see represent chains of amino acids. There are about 500 amino acids, which are difficult to draw. So you often see ribbons or tubes drawn instead. The 4 blue disks represent the "heme" groups that are in the hemoglobin protein. The "heme" groups gives blood its red color. Oxygen and carbon dioxide are held in place with help from the iron atom in the center of the heme molecule; however, if carbon monoxide is inhaled, it will stick to the heme groups more strongly than oxygen or carbon dioxide. So that hurts the body in two ways. It prevents the red blood cells from carrying oxygen and from getting rid of carbon dioxide waste. So you suffocate from lack of oxygen and you build up high levels of carbon dioxide, which makes the blood acidic. |
|
Unsafe levels of carbon monoxide is more than 9 parts per million (9 ppm) which means 9 carbon monoxide molecules for every one million air (oxygen and nitrogen) molecules. One way of visualizing that is to picture a cup of carbon monoxide released inside your home. That amount is safe, but any more than one cup of carbon monoxide that is placed in your home becomes unsafe. So it doesn't take much carbon monoxide to be dangerous. A typical burner on the stove can make that much carbon monoxide in a few seconds if the flame gets starved of oxygen. |
| To bring awareness to carbon monoxide poisoning, students in an university in England made the below poster. It's appropriate that they are laying down with eyes closed because victims of carbon monoxide poisoning often go unconscious and never wake up. |
|
Other Toxic Gases |
 |
Sulfur dioxide (SO2) is created when sulfur is burned. There is a certain amount of sulfur in coal, diesel, and gasoline. So when any of these are burned, we get sulfur dioxide. Under certain conditions sulfur dioxide adds an oxygen to form sulfur trioxide (SO3). In contact with water, sulfur trioxide become sulfuric acid. This is the source of acid rain. If you breath sulfur trioxide, sulfuric acid will form in the lungs. The illustration also shows nitrogen oxides (NO2, NO, N2O, and more) which forms when nitrogen in the air is subjected to a lot of heat. NO2 become nitric acid (HNO3) when in contact with water. |
|
Formaldehyde (CH2O) is used to make all kinds of plastics, resins, and adhesives; unfortunately, when formaldehyde is used to make these products, a certain amount of formaldehyde does not get converted and remains in the product. For example, the glue used in carpeting and particle board releases formaldehyde as a gas for weeks and months. This can make people sick. One highly publicized case happened when victims of Hurricane Katrina were provided with new trailers as temporary living quarters. These newly manufactured trailers still had high levels of formaldehyde evaporating (outgassing) from the glues and resins used to build the trailers. That made people sick. This happened again when victims of a flood in Iowa in 2008 were given trailers. If you move into a new house or get new carpeting or cabinets, try to bring fresh air into the house regularly. The levels of formaldehyde coming from these products will gradually diminish over a few days, weeks, or months depending on the levels and temperature. |
|
New car smell. Everyone can identify a new car by it's "new car smell". Even though we take pride in the new car smell of our new car, most of the chemicals causing that smell are rather toxic. There are about 40 chemicals that a new car releases as vapor. Formaldehyde as just mentioned is one of them. Plastics are usually safe but the chemicals that make plastics are not so safe, and there are usually residues of those chemicals still in the plastic. There are also phthalates, which are placed in plastics to make them more flexible. Those can also evaporate. Again, fresh air should be brought into a new car especially for the first month to reduce the concentration of these unhealthy chemicals. |
|
Pollution in the air can consist of many things. In this tutorial, we covered some of the more common ones.
The image is of Phoenix during one of its bad air days. |