
| <-Chemistryland Home |
|
|
| Our world is not wrapped in plastic, but it sure contains a lot of plastics (also called polymers) both natural and man-made. The beauty of polymers is that you can build so much with just simple building blocks. | |
|
What
does polymer mean?
|
|
 |
"Poly" means "many" and "mer" means "parts." So "polymer" means "many parts." The parts are usually the same part used repeatedly in a chain-like manner. Polymers are also referred to as plastics because they are easily molded. |
 |
Polymers are not just a human invention. Nature has many examples of polymers. Cotton fibers are made of sugar molecules that are repeated in a chain-like manner. Hair, wool, and other natural fibers are polymers. They are made by chaining one or two substances one after another. |
 |
It's often easy to see if something could become a polymer. From the shape of these parts, you can probably guess how they might stick together. |
 |
That's right. The rounded end will connect to the open end. This approach is used by many toys that let you build things. It is also used in chemistry to build things. |
 |
Now consider these parts. At first glance there doesn't seem to be a way for them to connect with each other. |
| Now be aware that one of the connecting sticks can spring open. To see animation of this, move cursor over the image. As long as cursor is over image, animation repeats. | |
| 1) We start with several pairs of balls each with two connecting sticks. 2) Something causes one of the connecting sticks to fling open. It then connects to another ball, but in doing so, causes one of its connecting sticks to fling open. 3) In moments all of the pairs of balls are now connected. Move cursor over image to see animation. | |
|
Chemistry compounds that tends to connect
in chains (polymerize).
|
|
|
Before I show you the compounds
that like to polymerize (meaning to become polymers), let's look a couple
of compounds you are already familiar with. Below on the left is methane
(natural gas). It is made of one carbon and four hydrogens. The top drawing
is the typical atoms "as balls" drawing. The bottom drawing
shows the electrons that are involved in keeping the atoms together. The
"H" with a plus sign is hydrogen because it has one proton (a
plus charge). The small white ball with a minus sign is the electron from
the hydrogen. Carbon has 4 outer electrons shown as small gray balls.
Carbon, like most atoms, are more stable with 8 electrons around it. The
4 hydrogens share 4 electrons and carbon has 4 of its own. So this is
a stable configuration. Let's now look at propane...
|
|
 |
|
| Propane has 3 carbon atoms. The carbon atoms stay together because they share one electron of their own with their neighbor and borrow one electron from their neighbor. This sharing holds them together and creates the stable configuration of 8 outer electrons. | |
 |
This is ethylene. It is also a gas. It has two carbons and 4 hydrogens. What's different is the two carbons are held together because they share two electrons each instead of just one, like in propane. The drawing on the right is sometimes used to indicate that each carbon is sharing two electrons each. They call this a double-bond. This should remind you of our earlier balls with two connector sticks. Remember how one of the sticks would spring open to connect to other balls? Ethylene does something similar. |
 |
Sometimes heat, high energy light, or something else causes the double-bond to break and two of the middle 4 electrons split up and end up on the two outer ends of the molecule. These electrons are unpaired, which makes them eager to join with another electron. So if they bump into another ethylene molecule a chain reaction starts... (place cursor over the image to see animated version) |
| The unpaired electron triggers the ethylene molecule that bumps into it, to shift the inside electron to pair with it. That then causes the newly unpaired inner electron to move to the outside and it is now an unpaired electron ready to cause the next ethylene molecule to repeat the process. This happens quickly and in moments thousands of these ethylene molecules are now attached and a polymer is formed. The name of this polymer is appropriately called, polyethylene. | |
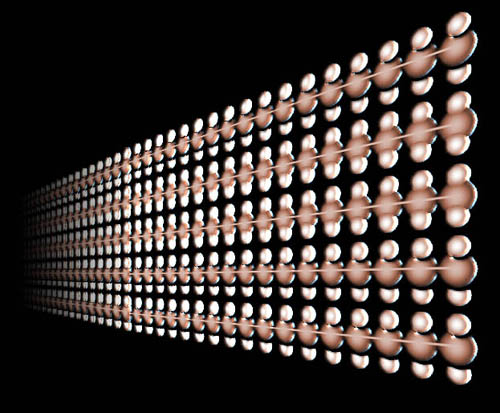 |
Polyethylene is the most common of all polymers. There are two types of polyethylene polymers (plastics). One is when the polyethylene exists as long straight chains. The picture here shows the chains of one carbon with two hydrogen atoms repeating. The chain can be as long as 20,000 carbons to 35,000 carbons. This is called high density polyethylene (HDPE). Sometimes they are made up to 500,000 carbons long. |
 |
Again polyethylene is the most common plastic you see. High density polyethylene HDPE is used for bottles, buckets, jugs, containers, toys, even synthetic lumber, and many other things. |
 |
Sometimes the chains get up to 500,000 carbons long. Here
they are tough enough for synthetic ice, replacement joints and bullet-proof
vests. This is called Ultra High
Molecular Weight
PolyEthylene
or UHMWPE. |
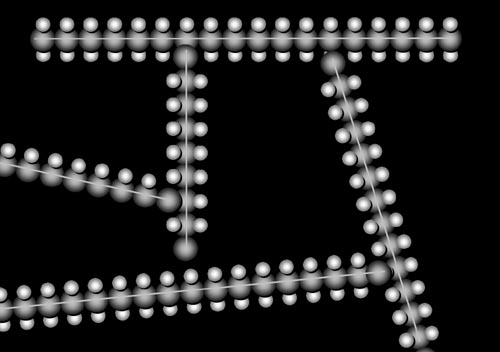 |
We've mentioned high density polyethylene, you probably were thinking, there must be low density polyethylene (LDPE). You are correct. It is made by causing the long chains of ethylene to branch. That way they cannot lie next each other, which reduces the density of the polyethylene. This makes the plastic lighter and more flexible. |
 |
Low density polyethylene is used to make plastic bags, plastic wrap, and squeeze bottles, plus many other things. |
| There are many types of plastics, but they all are based on taking one or two small molecules and starting a chain reaction that connects hundreds or thousands of these small molecules into long chains or branching chains. By controlling the length and the branching, you can control the final hardness or flexibility of the polymer plus qualities like resistance to solvents, acids, or heat. | |
|
CONSEQUENCES OF MAKING
& USING PLASTICS
|
|
 |
The favorite properties of plastics are that they are inert and won't react with what is stored in them. They also are durable and won't easily decay, dissolve, or break apart. These are great qualities for things you keep, but when you throw them away, they won't decompose. |
 |
The answer is to recycle the plastics. Here we see a bunch of CDs getting recycled. |
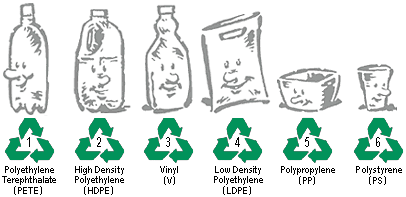  |
Here are two recycle code drawings. You already know about HDPE and LDPE. The others are similar to polyethylene except they have additional molecules attached to the polyethylene to give it different properties. For the recycling to be efficient, it is important to separate them into their perspective category. |
| I'm always happy to see trash turned into something useful. While at Yellowstone, I discovered they had made the boardwalk out of recycled plastic. | |
 |
|
| As I hunted for products made from recycled plastic. I found a lot of examples of tables, chairs, decks, etc. made from recycled plastic. I also found roofing material and pavers made from recycled plastic. There is also a "fleece" made from recycle soda bottles. This "fleece is made into clothes like the jacket below. | |
 |
|
|
It was encouraging to see a lot of companies
on the Internet selling products make from recycled plastic. Hopefully,
the consequences of using so much plastic won't be too much of a burden
on the environment. (Teachers: click
here to download a PowerPoint presentation that covers much of the
content above)
|
|