|
To evaluate Chemistry and Society even
in historical times, let's use these 5 steps.
|
|
|
1. What was the problem?
2. How did chemistry solve the problem?
3. What was the positive impact of solving this problem on society?
4. What were the negative
consequences to society and environment that resulted from this solution?
5. What chemistry principles need to be learned to better understand steps
1 through 4?
|
|
1.
What was the problem?
|
|
|
The problem was the storage of food.
During times of plenty, there is no need to store food or water, but during
a shortage having stored quantities of food and water would be critical.
Baskets had it use but baskets couldn't keep pests out of the food. Baskets
also were not waterproof. A container that could hold liquids or solids
was needed.
|
|
|
If food wasn't stored, then making it through the harsh
winters was extremely difficult.
|
|
|
If water wasn't stored, then it was hard to get through
the dry summers as well. This was true for animals and humans.
|
|
|
Whatever food you tried to keep was at
the risk of being eaten by other animals. Mice and rodents were just one
example.
|
 |
Insects like locusts could also devour any
food being grown or any fresh picked plants. |
 |
Other insects would get into any food stored
in the open or in baskets. |
|
|
Containers made from gourds were used
to store food, and animal hides sealed with beeswax were used to store
water. Neither were too durable or could keep out many pests.
|
|
2. How did chemistry
solve the problem?
|
|
The solution was in two parts. Learning how to make fire,
then learning how to make containers that could protect food. |
|
|
Primitive humans had to learn
that fire had important uses.
-Warmth-
-Softens food and makes it easier to digest-
-Makes food safer to eat-
-Scares away predators-
-It causes changes in materials that are placed in the fire-
|
|
|
Until man learned how to make fire, he had to depend
on lightning strikes to start a fire. Then the tribepeople had to try
to keep the fire going.
Mastering the chemical reaction called combustion would
result in a huge array of opportunities for humans.
|
|
|
Knowing how to make fire was a huge leap for humans.
The tools were a stick, a plank of wood, a rock, bow with twine, and dry
tender. Moving the bow back and forth spun the stick that caused friction
on the plank of wood. The notch allowed embers that formed to drop onto
the dry tender. One movie that depicts this discovery well is Quest for
Fire.
|
|
|
Even Tom Hanks in the movie, "Castaway,"
rejoiced when he created fire because he knew all that it could do for him.
There are some cultures even today prefer the old method of using friction
to start a fire. |
|
|
Once you had fire whenever you wanted,
primitive man would naturally try throwing things into the fire. Sometimes
unexpected things happened.
Sometimes dirt that normally would turn to mud when it got wet, would
no longer turn to mud, if it had gotten subjected to fire.
|
 |
Dirt
that did this was pretty easy to recognize. After rains and after the ground
dried, this special dirt had cracks in it. The kind of dirt is called clay.
Clay absorbs water and swells. When it dries it shrinks, which causes the
cracking. |
|
|
When clay is wet, it is easily molded. When
it dries, it will hold its shape. However, if it gets wet again, it will
get soft again and lose its shape. So by itself it isn't very useful as
a storage container. However, being subjected to fire changes all of that. |
 |
When clay is subjected to fire or high temperatures,
chemical changes take place that makes the clay keep its shape even if it
gets wet again. It is now useful for storing food because wet weather won't
cause it to dissolve. With a good lid or stopper, it can keep out insects
and rodents because they can't eat through the fired clay. |
|
3. What was the positive
impact of solving this problem on society?
|
 |
The impact of creating containers that could withstand
the weather and keep out pests gave humans a huge advantage over other
animals.
Pottery making is a technology that nearly all cultures
mastered, which helped them survive the harsher seasons and periods of
little food or game. Without this practical use of chemistry, humans may
have gone extinct.
|
| The impact of learning how to make fire goes
far beyond just the making of pottery. This impact will be covered in other
tutorials. |
| 4. What were the negative
consequences to society and environment that resulted from this solution?
|
|
|
Whenever
there is fire using wood or dung, there is also some pollution. So during the firing of clay,
there would be smoke and pollution to the air. However, in primitive times
this was not done on a large scale, so the consequences were minimal.
|
| 5. What chemistry principles need to be learned to better
understand steps 1 through 4? |
|
|
|
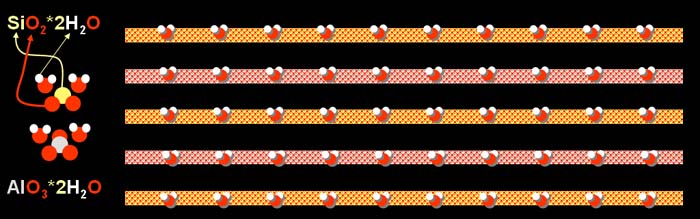
Clay is made up of layers. This particular clay has one
layer (top layer in picture) that is made up of a stacking of one (yellow)
silicon atom (Si) bonded to two (red) oxygen atoms (O2)
which makes a silicon oxide molecule. That is bonded to two water molecules
(2H2O). Note that this water bonded to the silicon
and oxygen does not evaporate in normal drying and does not make the clay
look wet.
The second layer is made up from stacking one aluminum
atom bonded with three oxygen atoms and that aluminum oxide molecule is
bonded with two water molecules (2H2O). Again,
the water stacked with the aluminum and oxygen does not evaporate or make
the clay look wet.
|
|
|
| When water is added to the clay, the small water molecules
get in between the layers of clay. They will then act like little ball bearings
that allow the clay layers to slide back and forth. This gives the clay
its ability to be molded when wet. When dried, these water molecules evaporate
away and the clay gets stiff again. The water molecules that are embedded
in the layers with the silicon oxide and aluminum oxide do not leave during
normal drying. |
|
|
|
Upon heating, the water that was added to the clay leaves
first (blue arrows). At the point the clay is back to the way it was before.
However, upon more intense heating the water molecules that were bonded
to the silicon oxide and aluminum oxides in the layers begin to be driven
off (purple arrows). After these water molecules leave, the clay will
not reabsorb the water molecules. In other words the clay upon getting
wet again will not become soft.
Also at the time the water is turning to steam and is
driven off, the clay can break because of trapped steam. Early on potters
learned that a little sand or ground pieces of shells added to the clay
would allow a path for the water vapor to escape and therefore reducing
breakage of the clay. This material added to allow water vapor to escape
is called "temper." Below the two irregular items that stick
through the layers represent the two temper materials, sand and ground
shells.
|
|
|
As the clay loses the water molecules that was bonded
to the silicon and aluminum oxides, the clay shrinks and the space between
the layers disappear. On continued intense heating, the silicon and aluminum
start to bond to each other. A new mineral appears, and it grows in needles
or filaments (jumbled straight lines in picture). This fibrous mineral is
called mullite and the formula is Al6Si2O13,
which means that it stacks in a way that repeats 6 aluminum atoms, 2 silicon
atoms, and 13 oxygen atoms. The good news is that these fibers help bind
the layers together and give the fired clay strength.
To download a PowerPoint file with an animation of the above
steps click here. Note this requires
PowerPoint found in Office XP (which is PowerPoint 2002) or PowerPoint 2003,
or a plug-in that allows Internet Explorer to run the PowerPoint. The plug-in
can be downloaded and then run (double-clicked) to install. It's 3.6 megabytes
and only works on Windows computers and only with Internet Explorere 5.0
or higher. Click here to download the plug-in. |