#1: From the salad, you are acquainted with the building blocks for the elements at a personal level. Match the atomic particle to the correct sense.
1. Protons
2. Electrons
3. Neutrons.
Senses: Sight, Taste, Pressure (from weight)

#3. In the picture of the helium atom to
the right, we see the electrons staying away from each other, but the
protons (+) seem to disobey the rule about like charges repelling. How
is it that protons will sit up against each other in the nucleus of an
atom?


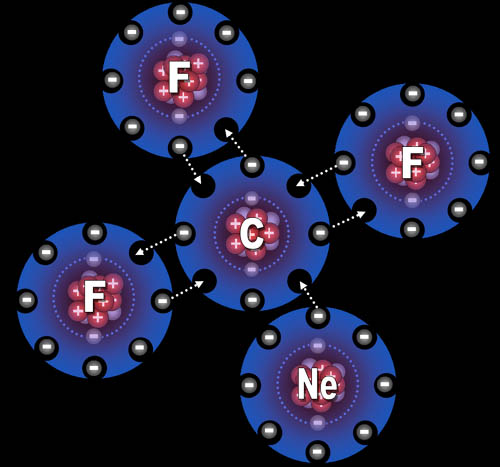
#5. The biggest factor regarding elements'
behavior comes from the outer electrons. In the picture we see three fluorine
atoms ready to combine with carbon. What do you think the neon atom will
do, since it has eight outer electrons?