
General Chemistry I
Instructor: Ken Costello
Spring 2006:
Room PS2S:
Tue/Thur 7:30-8:45am
 |
CHM-151 Lecture Homepage:
General Chemistry I |
Instructor: Ken Costello Spring 2006: Room PS2S: Tue/Thur 7:30-8:45am |
|
The PowerPoints you download are good for review. But most do not have a narration or captions. To see animations, you need PowerPoint 2002 (from Office XP) or PowerPoint 2003 (from Office 11) or download the Windows PowerPoint viewer (click here for viewer 1.86 Megs). The new Office for Mac should work, but I haven't test it yet. To Download click with right mouse button and choose "Save Link Target as..." However, if you just click on the link and have PowerPoint on your computer, the presentation may start downloading in the background (no message) and it might take awhile before you see the PowerPoint presentation popup. |
|
|
SYLLABUS:
If you just want to refer to the syllabus, click
here. If you want to download the syllabus presentation shown in class, click here. (14 Megs). |
|
HOMEWORK ASSIGNMENTS,
HANDOUTS, PRACTICE TESTS, & TAKE-HOME TESTS To check grades click here. |
|
|
THREE BARRIERS TO LEARNING: This is the presentation given the second day of class. It is about the Three Barriers to Learning. Also, the videos used in the presentation are not here because of their size. Size is 3.5 Megs Click here to download |
 |
|
IT'S ALL ABOUT BUILDING BLOCKS: Chemistry
is made simpler when you realize it is all about building blocks. You
can download PowerPoint this is sometimes shown in class, but you will
get more explanation going through the six online tutorials listed
below.
This
is also a
homework assignment. Go
to homework page. |
 |
|
EARLY USES OF CHEMISTRY: |
 |
|
WHAT'S IN A NAME? |
|
|
WHAT ARE IONIC COMPOUNDS? This PowerPoint explains what "ions" are plus helps in understanding the charges that atoms have. This knowledge is useful in naming ionic compounds. Download (629 K). |
|
|
NAMING IONIC COMPOUNDS This PowerPoint gives details on naming ionic compounds. Ionic compounds made from two elements (binary compounds) and those that use polyatomic ions are covered. Download (915 K). |
|
POLYATOMIC IONS & WHERE THEY ARE FOUND This PowerPoint shows how polyatomic ions
are related to binary covalent compounds (e.g., non-metal gases). Lewis
structures are used to help explain the charge on the polyatomic ions.
Where you see compounds with polyatomic ions is also covered. |
|
|
NAMING AQUEOUS ACIDS AND THEIR SALTS This PowerPoint gives details on naming acids based on their salt name. This covers both binary acids (e.g., HCl, HBr) and ternary acids (e.g. HNO3, H2SO4, etc.) Download (216 K). |
|
|
CHAOS TO ORDER: The development of the Periodic Table of the Elements Humans have always fought against chaos. The development
of the Periodic table tracks the efforts to understand and organize the
chaos. The table of elements started with just one element (water), then
four elements, up to the present table that has well over a 100 elements. |
 |
|
WHERE DID THE ELEMENTS COME FROM? Download (12 Megs) |
|
|
THE MAGIC OF METRICS This PowerPoint first points out the complexity of the English measurement system, which is why the metric system was invented. How metrics was invented is covered plus how it simplifies all of our measurements. Download (6.3 Megs). |
|
CHEMICAL REACTIONS: Chemistry's Target This presentation looks at the types of chemical reactions: Synthesis, Decomposition, Single Replacement, Double Replacement, & Combustion (oxidation). To demonstrate balancing, it uses the manufacture of bicycles, tricycles, and wagons as an analogy of how atoms are accounted for when balancing chemical equations. Download (1.3 Megs) |
|
|
SPECIFIC HEAT A gram of water requires one calorie of energy to raise one degree. Other materials have their own specific amount of heat to be raised one degree. This property is called Specific Heat. This presentation also covers how this knowledge is useful. Download (2.5 Megs) |
|
|
THE GOOD NEWS & BAD NEWS OF CHEMISTRY: Size is 2,368 kilobytes (2.3 Megs) |
 |
|
SOLUBILITY, CONCENTRATION, & MOLARITY Size is 3,861 kilobytes (3.8 Megs). Click to download. |
|
|
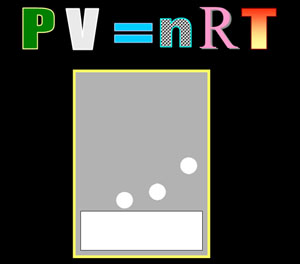
IDEAL GAS LAW: This presentation covers the history of gases and how they were put to work. It also covers how the PV=nRT formula came about. P=pressure, V=volume, n=moles, R=conversion constant, T=temperature. Counting molecules of gases is impossible, but they can be counted using the PV=nRT formula. Size is 8,493 kilobytes (8.4 Megs) To download click here. |
 |
COLLIGATIVE PROPERTIES: Get in the way and create disorder. This presentation is an introduction to colligative properties.
No math is covered, but interesting examples are shown. |
|