
To the left is methane, which is natural gas. One carbon and four hydrogen atoms make up methane. This is another example of electrons being shared by atoms. Shown are just 3 electrons, not all 10 that are there. But you can still see how they move around and are shared by different atoms. This kind of bonding is called covalent bonding. "Valence" comes from a Latin word meaning "capacity and power." The valence electrons give the atoms the capacity and power to combine (bond) with other atoms. Here the valence of carbon is 4, and hydrogen is 1. That's how many electrons are involved in bonding.
Compounds are often classified as Organic
and Inorganic.
In the below image, the rocks, water, and soil contain inorganic
compounds, and the tiger, grass, and trees contain organic
compounds. You can think of organic compouds
as those more likely found in organisms.
Inorganic compounds, in contrast, are those
created by forces within the Earth. Minerals,
for example would be inorganic compounds.

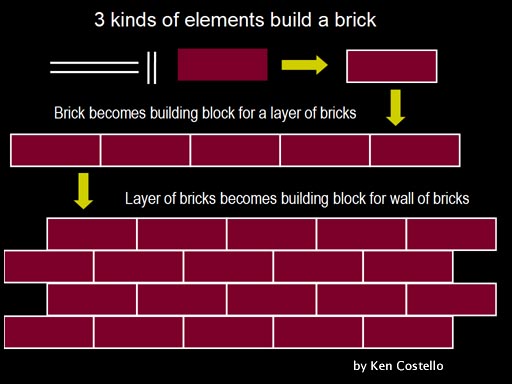
When we look at what things are made of, we find that elements usually make a small compound that is then used as a building block for larger compounds. These larger compounds are then used as building blocks for even larger compounds. It's an efficient approach. An analogy would be to draw a brick with 2 long lines, 2 short lines, and a red rectangle (these are the elements). These elements, bonded together, represent a small compound (1 brick) that is used to build a larger compound (layer of bricks). The layer of bricks becomes the building block for a wall of bricks.

