|
|
Inorganic
compounds are those substances that do not ordinarily come from living things.
For example, the cement in Hoover Dam, the metal used to help build the
dam, and the surrounding rocks are not produced by living organisms. The
water, also, is usually considered an inorganic compound even though living
things need it. The asphalt highway, however, contains organic compounds
because the asphalt came from petroleum, which is a decomposition product
of ancient forests or algae. |
|
Oxygen
46%
Silicon 28%
Aluminum 8%
Iron 6%
Calcium 4%
Sodium 2.4%
Magnesium 2.3%
Potassium 2.1%
Titanium 0.6%
Hydrogen 0.14%
|
To better understand the building blocks of inorganic
compounds, let's look at the 10 most abundant elements in the Earth's
crust. Oxygen is the most abundant by far, so you would expect oxygen
to be involved in building blocks for inorganic compounds. Silicon is
the second most common, so it's not surprising that silicon bonded with
oxygen is a very common building block.
|
| |
When you look at a mountain, be aware that
about 50% of it is oxygen and when combined
with silicon, the two make up 75% of that mountain.
Silicon usually bonds to two oxygens. If this building block repeats,
you get quartz- a very common mineral. The name for silicon with two oxygens
is silicon dioxide (silica). This is also the main ingredient of glass. The difference
between quartz and glass is that in quartz the stacking of silicon dioxide
is regular and forms a crystal. In glass, the stacking of silicon dioxide
is irregular (non-crystalline) plus they often add other inorganic compounds to glass to get it to melt easier.
|
|
|
The
formula for silicon dioxide is SiO2. Silicon is actually bonded
to four oxygens, but those oxygens are bonded to other silicon atoms. So the
average is one silicon for every two oxygen atoms. My graphic is trying
to show the 3-dimensional stacking of silicon dioxide. |
|
Common
metal
|
Chemical name when combined with
silicon and oxygen (silicate)
|
Mineral name
|
|
Aluminum
Iron
Calcium
Sodium
Magnesium
Potassium
Titanium
|
Aluminum
silicate
Iron silicate
Calcium silicate
(calcium iron silicate)
Sodium silicate
Magnesium silicate
Potassium silicate
Titanium silicate
(titanium calcium silicate)
|
Kyanite
& Sillimanite
Fayanite
Andradite (if with iron)
Water Glass and Silica Gel
Fosterite
Silica Gel
Titanite (if with calcium) |
|
"Silicates"
is a general term for minerals that have silicon
and oxygen, but also may contain a metal or
sometimes hydrogen. They are the largest class of minerals.To the left are
the metals listed in the top 10 most abundant elements. Each one of them
can be bonded with silicon and oxygen. Just say aluminum silicate, iron
silicate, calcium silicate, and you are giving the chemical
name for many minerals. For example, aluminum silicate Al2SiO5
is the mineral known as kyanite. Remember that most minerals were named before they knew what elements were in them. So mineral names often reflect their color, the person who discovered it, or some other trait rather than their chemical composition. |
|
|
Aluminum
is the third most common element in Earth's crust, so you would expect aluminum
and oxygen to bond and form building blocks. It's called aluminum oxide
(Al2O3). The repeating pattern is 2 aluminum atoms
and 3 oxygen atoms. The mineral, corundum, is made of aluminum oxide. It
is very hard, which is why it's used in sand paper. |
|
|
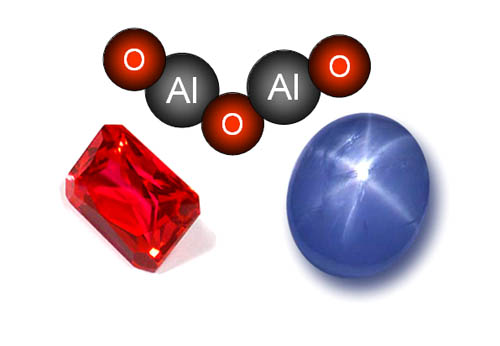
Because of its hardness, aluminum oxide is found in gemstones,
which are valuable because they resist scratches. Aluminum oxide is the
main building block for rubies and sapphires. Rubies have a trace amount
of chromium, which gives it the red color. Sapphires are also built from
aluminum oxide, but trace amounts of other metals give it different colors.
|
|
|
The three most common elements are combined with other
elements to build many minerals. Topaz is (AlF)2SiO4
meaning "Aluminum and fluorine is bonded together, and a pair of
them is bonded to one silicon and four oxygens atoms." This is the
repeating building block for topaz.
Emeralds are basically the same but the metal beryllium
(Be) replaces the fluorine (F).
|
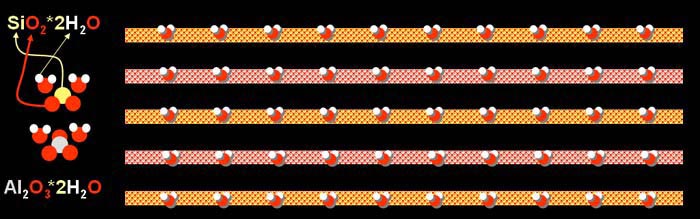
| In an earlier tutorial on the
history of chemistry, you learned about clay chemistry. Below is an image
from that tutorial. It should not surprise you now, that the common soil
known as clay is made from oxygen, silicon, and aluminum (the 3 most abundance
elements). In clay, silicon dioxide and the aluminum oxide have water molecules
bound to them. |
|
|
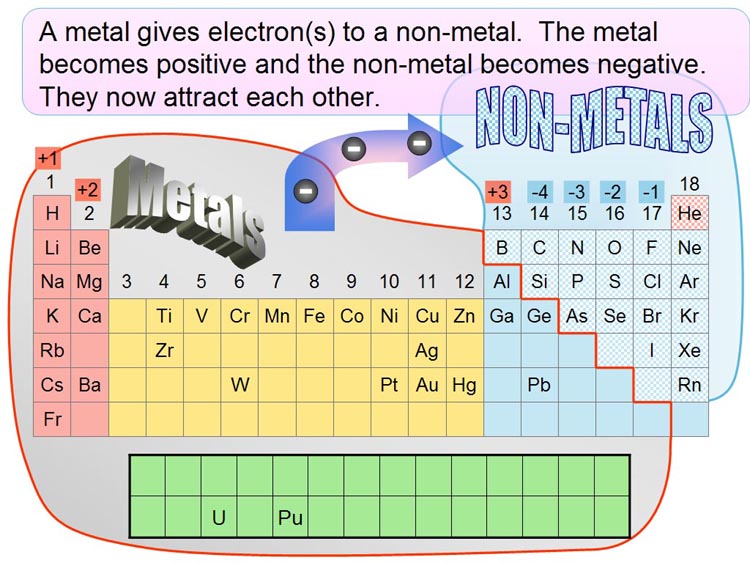
| The below table is a simplified
version of the Periodic Table of the Elements. I've included just the elements
you might have heard of. The left portion of the table are metals. The upper
right corner are non-metals. The pattern of an inorganic building block
is that a metal will combine with a non-metal (except for those in column
18 which are the noble [inert] gases). The reason is that non-metals (except
for noble gases) are a one to four electrons short of filling their outer
orbit. The metals, on the other hand, have one to four electrons loosely
held in their outer orbit. So it works out nicely. The non-metals pull electrons
off of the metal atom, leaving the metal atom positively charged. The non-metal
now has extra electron(s) that make it negatively charged. The now hold
together (bond) because of their opposite charges. |
|
|
|
|
On the chart, find "Na" in column
1 (also called, "Groups"). "Na" is sodium. On the right,
the non-metal, "Cl," is chlorine. They combine to form sodium
chloride (common table salt). Sodium also combines with fluorine (F) just
above chlorine to form sodium fluoride, which is what they put in toothpaste
to fight cavities. |
 |
In the Earth's crust, all metals
except gold and sometimes platinum, are found bonded to oxygen. The metal bonded to oxygen is the
ore for that metal. For example, you don't find copper metal in the ground,
but you do find copper oxides, which is copper combined with oxygen. So metals
combined with oxygen are other examples of inorganic compounds. |