Section 3224: Monday 11:15am-2pm
Instructor: Ken Costello


As you know we are bombarded by advertising of products which claim to be the best. All of this is just talk unless someone does some tests. This is why an organization like Consumer Reports is popular. It submits these products to scientific testing and gives us answers rather than just opinions.
When you learn laboratory skills, you will also be able to answer questions rather than debating them.

In all my chemistry courses, I like to divide a chemistry topic (or lab exercise) into three areas of focus making it easier to understand.
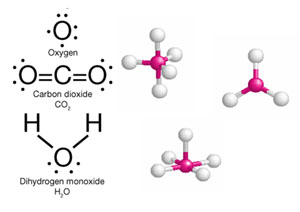
The building blocks focus sees chemicals coming from simpler building blocks.
Chemistry also involves force and energy. For example, the attraction and repulsion forces of + & - charges guide the assembly of atoms and chemicals. Energy is involved to overcome forces or is created by forces.
The third part of chemistry involves mathematics. The Earth represents the Metric system which is based on Earth's measurements and water.


Textbook: CHM151 General Chemistry I, Laboratory Manual by David Nachman.
You will also need a calculator.
Splash-proof safety goggles are required for every lab.


Here is some contact information:
Office: MC185
Office Hours: Tues/Thur 9-10:30am,
Wed. 11:15-12
Office Phone: (480) 461-7666
Departmental Secretary: (480) 461-7015
email: costello@mail.mc.maricopa.edu
or costello151@chemistryland.com
- Gain hands-on chemistry skills.
- Learn how to approach a chemistry topic as a blend of building blocks, force & energy, and mathematics.
- Learn chemical nomenclature.
- Learn how to write and balance chemical equations.
- Learn how to take accurate measurements and interpret results.
- Improve your chances of survival: Gain knowledge of chemical reactions, better avoidance of chemical dangers, better at improvising, better at solving problems, and better at critical thinking.
- Learn and practice the principles of Green Chemistry