Question 1: Carbon is an element that comes in different forms. If stacked in 3 sided pyramid shapes, it's a diamond. If stacked in flat six sided shapes, it is graphite (pencil "lead"). If there's no order to the stacking, then it's charcoal. When it's activated (see image) it can trap organic molecules.
Visit the below website and list the 9 things that it
filters out. (I don't need the percentages) Also, it mentions "turbidity."
What is that?
The Web page doesn't say the filter contains activated charcoal but the list of contaminants are mostly organic compounds and lead can be trapped by activated charcoal with fine pores, so I'm sure it is activated charcoal.

Question 2: The filter described in question one says it filters out "atrazine." Go to the below Web site and report what atrazine is used for. What is one of its dangers? The last paragraph says what you can do about it. What is that?

Question 3: If you were to put some ocean water into your purification column the activated charcoal would take out the fishy taste but not the saltiness, which is mostly from dissolved sodium and chlorine.
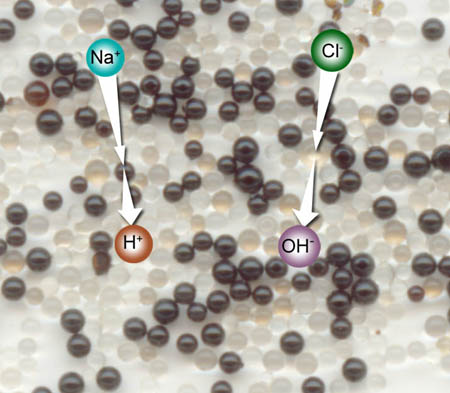
The deionizing resin that's in your column is made of two resins. I scanned this dual resin from your kit. When a positively charged sodium ion (Na+) touches a black resin sphere, it is trapped and a hydrogen [H+] ion takes its place. If the negatively charged chlorine ion touches a clear resin sphere a negatively charged hydroxide ion [OH-] take its place. When H+ and OH- comes together, what is formed? Hint: visit this link to Experiment 2 Lab 1 for image regarding this. Use your browser back arrow to get back here.
Question 4: You might wonder why would you care what the
pH of some liquid is. One reason is that acids attack and dissolve most
metals (many are toxic). The positive hydrogen ion [H+] tends to pull
off an electron from the metal, making the metal atom positive, which
causes water to pull the metal into the solution. If water is acidic,
iron pipes will dissolve. Fortunately, most pipes used in homes now are
copper, which resists acids. However, your copper pipes are soldered together
with solder made of tin and lead. From the chart, you can see that tin
and lead would be dissolved by acids in the water. In the home you would
be drinking water contaminated by lead. Some companies promote lead-free
solder using tin and antimony. Go to this site
http://householdproducts.nlm.nih.gov/
and do a search for "solder." Click on the product
named, "Hercules Swif 95 Lead Free 95/5 Tin Antimony Solder."
Report on some of the dangers of antimony. You will learn that many products
promoted as being safer (example, lead-free), may not be. Antimony is
just below Hydrogen in the chart, so even though it is very dangerous
to the plumber, it won't dissolve if the water is acidic. Check the "Oatey
Silver Lead-Free Solder" and see if that is any safer to use.
Lithium
potassium
calcium
sodium
magnesium
aluminum
zinc
chromium
iron
nickel
tin
lead
(Metals above hydrogen are dissolved by acid [H+])
HYDROGEN (metals below here are not dissolved by acid)
antimony
copper
mercury
silver
platinum
gold