| As usual, copy the questions below (highlight
the text, then press the CTRL key plus "c", or use the Edit menu
at top and choose "Copy")
In your email program start composing an email and paste
the questions to your email (use CTRL-V to paste or use Edit menu and
choose "Paste"). You can then answer them in your email and
send it to chm107pc@gmail.com.
|
#1. Using
the rule of LIKE DISSOLVES LIKE, which of the following left outside
would get dissolved by rain and end up in your well water.
Candle wax
Sugar
Salt (sodium chloride)
Drywall (CaSO4) (hint Ca=+2 charge,
SO4=-2 charge)
Lead Chloride
Sodium Fluoride
Plastic bottles
Glass bottles
Alcohol
|

|
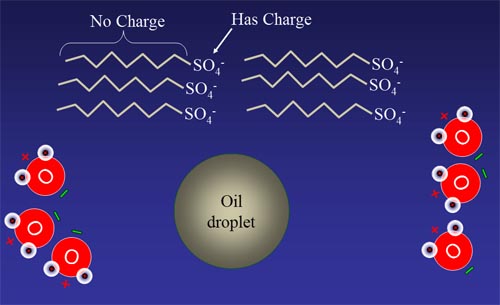
#2: Soap is made from a chain of carbon and hydrogen
atoms (zig-zag line) connected to a charged group of atoms like SO4-.
The chains of carbons have no charge so they are ignored by water as
they migrate through the water.
|
 |
|
#2 continued: The oil in the oil droplet
has no charge and blends nicely with the long carbon chain end of soap,
which also has no charge. The water molecules, however, are attracted
to the charged end of the soap molecule.
|

|
| #2 cont'd: Eventually, the soap molecules
will align themselves so the non-charged end will stay dissolved in the
oil drop, but the charged SO4- end well be held on
to by water. Instead of the oil drop getting squeeze out of the way of water,
it gets attracted by water because it has these charge SO4- groups sticking out of it. So in essense, the soap allows water to dissolve
oil. Question: Is the plus or negative side of water attracted to the SO4- groups? |
 |
| 3. Since water has a partially charged
plus and minus ends, it aligns itself like a crosslinked net (or skin) at
the surface of water. They call this surface tension. Mosquito larva take
advantage of the strength of surface tension by suspending themselves from
the water's surface. |
 |
| 3 continued. This represents what water
molecules would do at the surface. They line up + to -, which gives it strength.
However, if soap is added to the water... |
 |
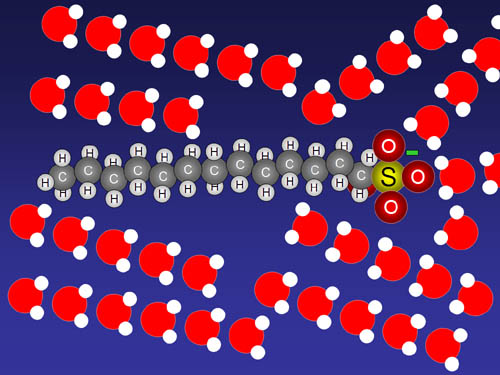
| ...the alignment of water is disrupted.
This soap molecule has a chain of carbons and hydrogens that water ignores,
and a negatively charged SO4- group that attracts the
positive end of water molecules. This disrupts the previous chain of water
molecules (the net) but the water still locks onto the negative SO4- group.
The long chain of carbons and hydrogens have no charge so water is not attracted
to these chains. This weakens the surface tension. Why? |
 |
| Soap added to water causes the mosquito
larvae to sink thereby dooming the adult mosquito from ever making it to
the air. As this remarkable picture shows, with water surface tension intact,
the adult mosquito can escape into the air. |
 |







