In your email program start composing an email and paste the questions to your email (use CTRL-V to paste or use Edit menu and choose "Paste"). You can then answer them in your email and send it to costello@chemistryland.com.
#1:
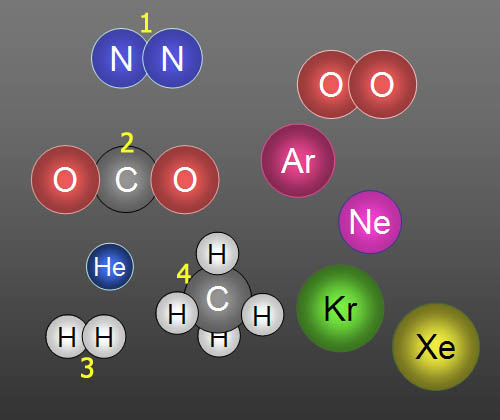
Identify molecules 1, 2, 3, & 4.

#3: Liquid nitrogen is used for a lot of
chemistry demos. This student has just dipped a rose into liquid nitrogen.
The next step is to drop the rose and watch it shatter like glass. It
even kind of sounds like glass breaking. Liquid oxygen is almost the same
temperature and could be used in these demos, but it's never used. Why?

#4: This is a hyperbaric oxygen pressure
chamber. Instead of air, the patient is surrounded by pure oxygen at a
pressure about 3 times that of normal air pressure. Since normal air is
20% oxygen, pure oxygen would be 5 times more oxygen, and at 3 times normal
air pressure, a patient gets 15 times more oxygen than normal. Do a Web
search and give at least 5 medical problems that hyperbaric oxygen therapy
treats.
