|
|
Chapter 1: “The Air We Breathe”
• Misconceptions
about air
• Composition of clean air
• Atoms and molecules
• Formulas and Chemical Names
• Chemical reactions
• Combustion
• Air pollutants
(Blue items covered in later
tutorials)
|
|
Composition
of Clean Air
|
|
|
|
In the fall of 2002 I got to go to Switzerland.
The morning of November 6th, I had to leave the village of Zermatt, but
wanted to take one more look at the Matterhorn. The air was absolutely
clear. Cleaner than I have ever seen air anywhere. The Matterhorn jumped
out at you. By the way, Zermatt does not allow automobiles. People walk
or ride electric vehicles. The train that goes there and the trolley that
takes you to the ski lifts also run off of electricity.
|
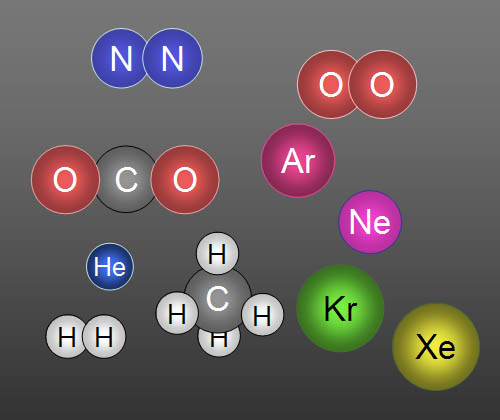 |
Here are 10 gases that make up clean air: In
order of highest to lowest concentration they are Nitrogen, Oxygen, Argon,
Carbon dioxide, Neon, Helium, Methane (CH4), Krypton,
Hydrogen, and Xenon. Five of them travel alone, so we call them atoms.
For example, a helium balloon contains atoms
of helium (He), but an oxygen tank contains molecules
of oxygen (O2). When there's two or more atoms are
bonded together, they are called molecules. |
|
|
NITROGEN
(N2) #1: 8 out of 10 atoms
(or molecules) of air are made of nitrogen. Nitrogen gas can't be seen
but when it is cooled to -320 °F (-195°C) it turns to a liquid,
which you can see. Being this cold makes it handy for many things. Shown
here, it is being used to quickly freeze cream and sugar to make ice cream.
|

|
NITROGEN #2:
Liquid nitrogen is also used to cool certain metals to the point where they
become supercondunctors. Superconductors conduct electricity (electrons)
without any resistance. In this picture a rectangular shaped magnet had
been dropped onto a metal disk. As the magnet fell toward the metal disk,
the magnetism in the magnet caused currents of electricity to flow inside
the metal disk. The currents created in the metal disk by the falling magnet
made that part of the metal disk a magnet itself. The falling magnet then
feels the repulsion of the magnet it just created. The falling magnet will
then stop just above the metal disk. Electricity continues to flow in the
metal disk constantly creating a mirror image magnet that repels the real
magnet. This will continue forever as long as the metal is cool enough to
stay a superconductor. |
|
|
NITROGEN #3: Oxygen reacts
with many things including paint. Therefore, expensive works of art and
other rare relics are stored in pure nitrogen. Nitrogen is fairly inert
and doesn't react (combine) with most other substances, so it is used
to protect items and keep oxygen away..
|
|
|
|
NITROGEN goes bad: Under
high temperatures, like in a jet engine or car engine, nitrogen will combine
with oxygen to form a class of toxic
compounds called nitrogen oxides. The simplest having one nitrogen and
one oxygen (NO). Others have two nitrogens and one oxygen (N2O),
one nitrogen and two oxygens (NO2), and the fourth
has two of each (N2O2). Their
names (in order) are nitrogen oxide, dinitrogen oxide, nitrogen dioxide,
and dinitrogen dioxide. Nitrogen dioxide has a brownish appearance and
is often what you see in polluted cities.
|
 |
Oxygen:
2 out of 10 atoms/molecules of air is oxygen. Oxygen is produced by plants
and is consumed by animals. Animals need oxygen to "burn" the
food they eat in order to get the calories (energy) they need. The way animals
use oxygen to burn food is different than a fire, but it produces the same
products of carbon dioxide and water. |
|
|
|
OXYGEN #2: Oxygen
is everywhere. As a gas it makes up 20% of the air. Some oxygen gas is
dissolved in water, and some oxygen gas is in the pores in the ground.
However, there's a lot more oxygen in this picture that we don't
normally consider. The weight of the water in the river is 90% oxygen
atoms. Remember water is H20. The one oxygen in H20 weighs 8 times
has much as the two hydrogen atoms combined. The trees and grass
are made of carbon, oxygen, hydrogen, and nitrogen, with oxygen accounting
for about half of a plant's weight. The rocks, the soil, and the
mountains contain metals and silicon that is bound to oxygen, which makes
up about 1/2 of their weight. Think about it; one half of a mountain's
weight is from oxygen. The only thing in the picture that doesn't contain
oxygen are the metal rails.
|
|
|
| OXYGEN #3:
In the movie Total Recall, there was an ancient alien factory that could
release the oxygen from the Martian soil. This is actually possible. Extreme
heat will cause the oxygen to separate from the metals and non-metals that
it was bound to. Most of Martian soil is iron oxide, which would turn to
iron metal and oxygen gas at high temperatures. In the pictures above, Arnold
presses the touch sensitive on "button" of the oxygen generator.
Next he gets blown out of the factory into the thin Martian air, which
causes the air in his body to expand. Fortunately, the oxygen rushes out
of the top of the mountain that housed the factory and they show Mars getting
an atmosphere similar to Earth's. Actually, this atmosphere would be pure
oxygen, which is not good to breath for long periods and would present extreme
fire hazards. It's lacking the 80% nitrogen. |
|
|
ARGON:
Argon is 1 out of 100 atoms/molecules of air. Argon is an inert gas that
is used to pressurize light bulbs. Being inert it doesn't react with the
tungsten metal, which makes the filament. The argon gas also helps keeps
the tungsten metal atoms on the surface of the filament from jumping off
the filament. |
|
|
CARBON DIOXIDE:
Carbon dioxide only makes 3 out of 10,000 atoms/molecules of air; however,
it supplies the carbon that plants use to make leaves, trunks, roots, etc.
It's hard to believe that the weight of giant redwood trees came from the
small amount of carbon dioxide gas in the air. |
 |
NEON:
2 out 100,000 atoms/molecules of air. Neon is a clear inert gas; however,
high voltage can strip electrons off neon atoms. As the electrons fall back
into place, they give off ultraviolet light (black light), which you can't
see. However, the glass tubes are coated with different phosphor powders,
which glow when ultraviolet light hits them. |
|
|
HELIUM:
5 out of 1,000,000 atoms or molecules of air are helium. Helium is the second
lightest gas (hydrogen is lightest). It is used to fill toy balloons and
weather balloons. It is so inert that there is no danger of igniting nor
will it combine with other elements. For this reason it is used in welding
by having helium gas surround an electric arc that is melting a metal. The
flow of helium gas keeps the oxygen away from the metal so that the oxygen
won't oxidize (rust) the metals being welded. |
|
|
| METHANE:
2 out of 1,000,000 atoms/molecules of air are methane molecules. Bacteria
is a big source of methane gas and this type of bacteria is found in termites
(see huge termite mound), cattle (cow flatulence), rice paddies, swamps
(called swamp or marsh gas), and in the sea bed. In the sea bed, methane
can be found trapped in ice (see person holding ice that's on fire). Methane
also comes from petroleum fields and is the natural gas you cook with. An
embarrassing source of methane is from the eating of beans. |
 |
KRYPTON:
1 out of 1,000,000 atoms/molecules of air is krypton gas. You may remember
krypton as the name of the planet that Superman came from. The pieces of
the planet was called kryptonite. The element krypton is used in lamps in
a similar way that argon is used (see above). The word krypton comes from
"Krypto" meaning hidden. The gas krypton was in a way hidden because
it took quite a while for scientists to discover it. |
 |
HYDROGEN:
1 out of 2,000,000 atoms/molecules of air is hydrogen. Hydrogen is the lightest
element, which is why is was the preferred gas to fill blimps and dirigibles.
Unfortunately, hydrogen is very flammable, which led to the catastrophic
tragedy of Germany's Hindenburg airship. |
|
|
XENON:
87 atoms out of 1,000,000,000 (billion) atoms/molecules of air are xenon.
Like argon and krypton, xenon is used as the gas in lamps. This one is for
a car's headlamp. These are probably the ones that look more blue. |
|
|
| WATER VAPOR:
The other gas we didn't list before is water vapor. The concentration of
the other gases are pretty consistent, but water vapor varies greatly. Using
a fish-eye lens, I took this picture of a double rainbow in Geneva, Switzerland.
It had rained all day, but just before the sun went down, the clouds parted
enough to allow the sunshine to strike the droplets of liquid water still
in the air. Water in vapor form (gas) is not visible. |
 |
One Last Look
at Compostion of Air: Imagine the volume of
air in a typical classroom that is 30 feet by 30 feet with 10 foot high
ceiling. Also assume, we somehow separated all the gases in the air. Oxygen
would cover the whole room to about 2 feet deep. Nitrogen in the air would
fill almost to the ceiling (another 8 feet minus a couple of inches). Argon
gas would fill another one inch layer over the whole room. The remaining
gases fill the last one inch. Carbon dioxide has about the same volume of
one student. Neon is 1.5 gallons. Helium would fill a one liter soda bottle.
Methane gas would fill someone's 1/2 liter water bottle. Krypton would fill
a 12 oz soda can. Hydrogen would fill about half of a 12 oz soda can. And
xenon gas would have the volume of a pencil's eraser. |



















