| Aluminum-13 | Berkelium-97 | Bromine-35 | Beryllium-4 | Nitrogen-7 | Fluorine-9 |
| Francium-87 | Iodine-53 | Krypton-36 | Gallium-31 | Mercury-80 | Molybdenum-42 |
| Sodium-11 | Samarium-62 | Strontium-38 | Titanium-22 | Tungsten-74 | Yttrium-39 |

The above image shows some of the setup I use when demonstrating the making of biodiesel. Now I also do the distillation and recovery of methanol but that's not shown in the above image.

Problem 1: How many liters is 48 fluid ounces? (Notice I said "fluid ounces" because ounces can also be a weight, where 16 oz equal 1 lb. However, this is not the weight called ounce.)

The recipe calls for 3.5 grams of sodium hydroxide (lye) per liter of oil. However, I did not use one liter of oil in the demonstration. I used 1.4 liters of oil.
Problem 2: So how many grams of sodium hydroxide (NaOH) do I need to weigh out?

Gasoline doesn't really freeze, but water in gasoline will. These bottles of methanol is used to dissolve water that might be in the gasoline. The recipe calls for 200mL of methanol per liter of oil. Problem 3: Again, I am using 1.4 liters oil, so how many mL of methanol do I need?
The bottle with an "X" is isopropyl alcohol and not methanol. Isopropyl alcohol will not work for making biodiesel. All bottles are 12 fluid ounces.
Problem 4: Does a 12 fl. oz. bottle of methanol have enough mL of methanol to fulfil the recipe for 1.4 liters of oil that you figured in problem 3?

Problem 5: What would those be?

Problem 6: How many 1 lb containers are needed? (The best way to do these problems is with dimensional analysis. Below is the set up.)
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
|
| 1 | |||||||||||
| 2 | 100 |
gallon | 3.785 |
Liters | 3.5 |
g NaOH | 1 |
one-lb jar | = |
??? | one-lb NaOH jars |
| 3 | 1 |
gallon | 1 |
Liter | 454 |
grams |
Problem 7: What formula goes in J2?

Here is both the ball and stick model of methanol and the space filled model. The ball and stick model makes it easier to see what atom is connected to what other atom. The space filled model shows the true size of the atoms. Methanol is also called methyl alcohol.
Problem 8a: What part of the molecule shown is the "methyl" group?
Problem 8b: What part of the molecule shown is the "alcohol" group?
Problem 9: What element is represented by the red spheres?
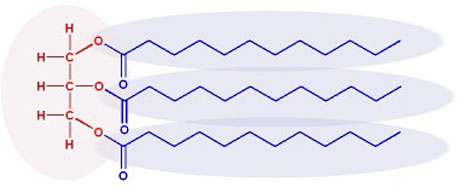
Here is a similar fat molecule but shown with the structural formula for the left 3 carbons and a skeletal formula for the long hydrocarbon chains.
Problem 10: Fats and oils are called triglycerides. Why are they called that?

Problem 11: Why does the word "glycerin"come from?
Problem 12: What is HOH and why did I color it the way I did?

Problem 13: What are the names of the following hydroxide compounds?
KOH
LiOH
Ca(OH)2
Mg(OH)2

Once in awhile the water and biodiesel will form an emulsion and not separate. The emulsion can last for days. To break up the emulsion, table salt can be added to the mixture.
When the oil is getting converted to biodiesel by the methanol/lye mixture, some of the oil is getting converted to soap by the lye. The soap will tie the water and biodiesel layers together to make an emulsion. The salt will interfere with the soap (like hard water does) and break up the emulsion.
Problem 14: Other salts could be used. What are the formulas for the following salts?
Potassium chloride
Calcium sulfate
Magnesium sulfate
Barium chloride

Remember, potassium hydroxide can be used instead of sodium hydroxide; however, the recipe of 3.5 grams of sodium hydroxide per liter of oil can't be used because potassium hydroxide weighs more per mole than sodium hydroxide. The active component is NaOH is the OH (hydroxide). KOH can be used but we have to have the same number of OH ions.
Problem 15: How many moles of OH are in 3.5 grams of NaOH? (hint: moles of OH is the same moles of NaOH since there's one OH per NaOH molecule.)
Problem 16: How many moles of OH are in 3.5 grams of KOH?
From the moles of NaOH in 3.5 grams of NaOH, use those moles to find the grams of KOH with that same number of moles. That is how people get the recipe for grams of KOH per liter of oil.

A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
P |
Q |
|
| 1 | Atomic # |
gal > liters |
rate in g/L NaOH |
convert g>moles |
Same moles |
mol KOH>g |
Adjusts for 90%w/w |
grams of 90% KOH |
|||||||||
| 2 | # |
gallon | 3.785 |
Liters | 3.5 |
g NaOH | 1 |
mole NaOH | 1 | mole KOH | 56.12 |
g pure KOH | 1 |
g of 90% KOH | = |
??? | grams of 90% KOH |
| 3 | 1 |
gallon | 1 |
Liter | 40 |
grams NaOH | 1 | mole NaOH | 1 |
mol KOH | 0.90 | g pure KOH | |||||
Problem 18: If making 50 gallons and using 95% KOH, which cells need to be updated?

The rule of like dissolves like is quite useful in the making and cleanup of biodiesel. One method of cleaning up the raw biodiesel is to use water. Here I am pouring water into the raw biodiesel. Earlier you saw biodiesel has long hydrocarbon chains similar to diesel. Those are non-polar so water is not attracted to those chains. That makes biodiesel not soluble in water. However, there are some residues in the biodiesel that we have to remove. They are NaOH, glycerin (CH2OH-CHOH-CH2OH), and methanol (CH3OH). Water (H2O or HOH) is attracted to all of these and they will all dissolve in water.
Problem 19: What do you see that is in common with all four of these compounds (water, lye, glycerin, and methanol)?
Problem 20: The density of biodiesel is about 0.86 g/mL. After mixing the water and biodiesel will separate. Which one will be at the bottom?

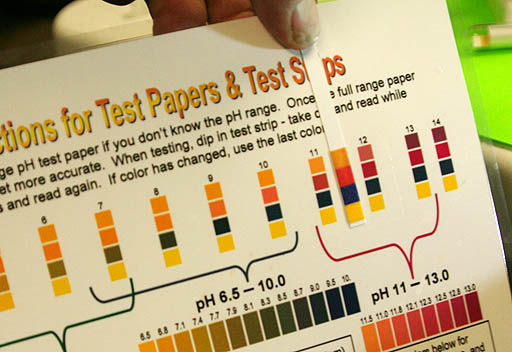
Here is the pH from the first wash of biodiesel. It's between pH 11 and pH 12. You are suppose to continue the washes until all of the NaOH is gone.
Problem 22: What will be the pH then?

Problem 23: What type of compounds neutralize acids?

Below is the equation for the neutralization of NaOH by the fatty acid shown. The fatty acid turns into soap.
Problem 24: What is the molar mass of the soap molecule?
NaOH + HOCO(CH2)10CH3 --> HOH + NaOCO(CH2)10CH3


I usually make up a liter of 0.5% w/v solution of NaOH. Other people use 0.1% w/v solutions, but I get more accuracy with 0.5%w/v.
Problem 25: How many grams of NaOH do you need to make one liter of 0.5% w/v NaOH solution (remember 0.5% w/v is 0.5g/100mL)?
I will then measure out 5 mL of the used oil. The problem with oil is that it won't mix with the NaOH solution that we will be adding to the oil. So I dissolve the oil in about 30mL of pure isopropyl alcohol. The alcohol dissolves both oil and water. That way the OH- in NaOH can get to the H+ in the fatty acids in the oil.


Let us say I fill the buret to the 20.0 mL mark with the 0.5% w/v NaOH solution. At the point the color changed, the level was then 16.6 mL. So it took 3.4 mL of my 0.5% w/v solution to neutralize the fatty acids in the 5 mL of oil. So now I need to neutralize the fatty acids in the batch of 20 gallons of used cooking oil that will get converted to biodiesel.
Problem 26: How many extra grams of NaOH powder is needed to neutralize the fatty acids in that 20 gallons of use cooking oil based on that titration (L2)? (See spreadsheet below for setup).
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
|
| 1 | Volume x concentration gives grams NaOH to neutralize the fatty acids in 5 mL of oil |
Divide by 5mL to make g NaOH needed per 1mL of oil |
This makes it g NaOH per gallon of oil |
times gallons makes grams |
|||||||||
| 2 | 3.4 |
mL NaOH | 0.50 |
g NaOH | 3785 |
mL |
20 |
gallons |
= |
??? |
g NaOH | ||
| 3 | 100.0 |
mL NaOH | 5.0 |
mL of oil | 1 |
gallon |
|||||||
Problem 27: This is the extra NaOH needed to neutralize the fatty acid in the oil. This is extra grams of NaOH. You still need to have 3.5 grams NaOH per liter. So what is the total grams of NaOH needed for these 20 gallons?
Problem 28: Other biodiesel producers use a 0.1% w/v NaOH solution and only titrate 1.0 mL of oil. So what cells above would need to be changed?

Methanol is used as a fuel in Indy Car racing (at least until 2007, then switched to ethanol). The oxygen in methanol (CH3OH) gives it some built in access to oxygen, which helps it burn faster. In biodiesel production, methanol is turned to methoxide, which attaches to the fatty acid side of the fat or oil. Because methanol is quite flammable biodiesel processing should avoid any open flames. Only electric or solar heating should be used.

Problem 29: The volume of that vat is 30 gallons. How many grams of methanol vapor are still in that vat? (Remember to use PV=nRT. Use algebra to solve for "n" which is moles of methanol. Pressure has to be in atmospheres [14.7 psi=1 atmosphere]. Change gallons to liters. Change Fahrenheit to Celsius and then to Kelvin. R=0.0821atm·L/mole·K. After you find "n" (moles) change that to grams using the molar mass of methanol, which is 32g/mol.

Problem 30: Assuming no loss of heat, how many minutes (cell T2) will it take for the 500 watt heater to heat up 100 gallons of oil that starts at 71°F and ends at 130°F? (See spreadsheet below for setup help if you want to use it)
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
P |
Q | T | U |
|
| 1 | Use density to get gallons to grams |
Specific heat of oil |
(130°F-71°)=59°F>°C |
÷ by watts so joules cancel H2 |
|||||||||||||||
| 2 | 100 |
gallons | 0.92 |
g | 3785 |
mL | 1.917 |
joules | =(59-32)*5/9 |
°C |
1 |
watt·sec | 1 |
min | = | ?? | min | ||
| 3 | mL | 1 |
gallon | g ·°C | 500 |
watts | 1 |
joule | 60 |
sec | |||||||||
Problem 32: Why did I divide by 500 watts rather than multiply by 500 watts?
Problem 33: A natural gas or propane heater would be faster in heating up the oil, but why are those not used when doing biodiesel production?

Problem 34: Are the bonds covalent or ionic bonds in methanol?


Problem 36: When sodium hydroxide is added to methanol, the negative OH- ion is attracted to what part of the methanol molecule?

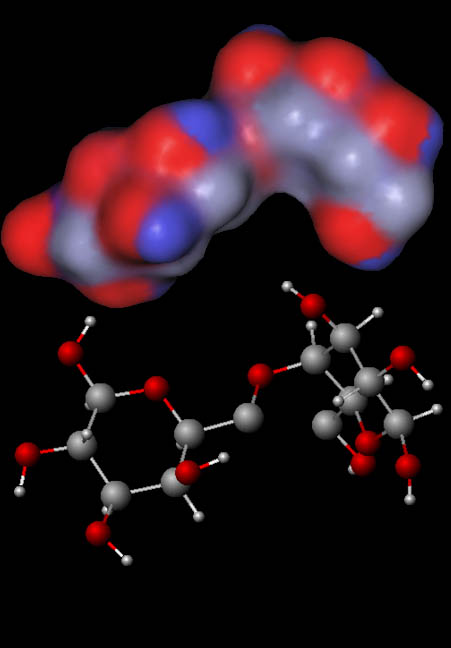
Here is a segment of cellulose, which is made from chains of glucose. Both ball and stick model and charge distribution models are shown. Paper towels (cellulose fibers) are often used to trap water that might be in the used cooking oil before the process begins. Cellulose is also used to dry the biodiesel after it gets washed with water.
Problem 38: Why does cellulose have a higher attraction to water than biodiesel?
Problem 39: What atoms show up as blue in the upper model?
Problem 40: What atoms are responsible for the grayish regions in the charge distribution model?
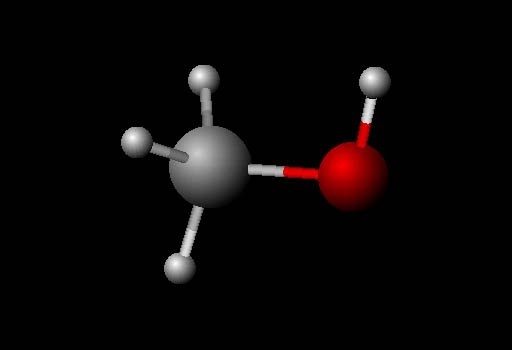
Problem 41: This is methanol molecule again. What kind of hybridized bonds are carbon using? (Choices are sp, sp2, sp3).
Problem 42: How many lone pairs does oxygen have?
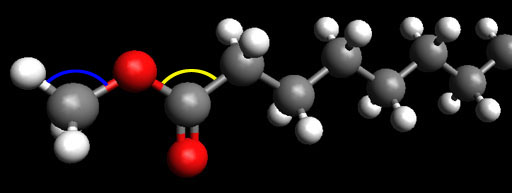
This is the end of biodiesel that has the carbon with the 2 oxygen atoms. That carbon is using what kind of hybridized bonds to connect to the 3 atoms that it is bonded to.
Problem 43: What bond angle (shown in yellow) is formed between the O-C-C?
Problem 44: What two atoms share a pi bond?
Problem 45: On the methyl group on the left, what bond angle (shown in blue) is formed between the H-C-O bonds?

After the reaction that creates biodiesel is finished, the glycerin is drained off, and then I recover the excess (unreacted) methanol. The boiling point of methanol is 65°C (148°F). So I heat up the biodiesel/methanol mixture to 65°C and the mixture boils. Methanol vapors go up to the bend and condense back to a liquid. Some liquid travels down the condenser (a tube within a tube) and then drips down in to the right side flask. Cold or cool water is run through the condenser to cool the vapors and liquid. See the below diagram for added clarity. There is still some glycerin in the mixture and we don't want that in the methanol. If its boiling point is considerably higher than that of methanol none will contaminate methanol recovered.
Problem 46: Look up and report the boiling point of glycerin.


My demonstration uses about half a gallon of biodiesel; however, most producers make 20 to 500 gallons at a time. I had a biodiesel co-op ask me about recovering 32 gallons of methanol from their 400 gallon biodiesel reactor tank. They needed a way to cool the methanol vapor. They wanted to use a 55 gallon barrel of water to cool the vapor and wondered if that was enough. That requires knowing the heat (enthalpy) of condensation of methanol. That value is the same as the heat of vaporization. Heat of vaporization needs energy (+ sign) and the heat of condensation releases energy (- sign). I searched for the both of them on the Internet because both values are the same. Here are the various values I found:
3340 Btu/gal, 506 Btu/lb, 1100 kJ/kg , 35278 kJ/kmol.
I want the value in calories because that makes the calculations with the water easier (1 calorie raises 1 grams of water 1 degree Celsius).
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
M |
N |
O |
P |
Q |
|
| 1 | Gallons methanol |
Heat of condensation |
Convert to calories |
||||||||||||
| 2 | 32 |
gallon | 3340 |
Btu | 262 |
calories | = |
??? |
calories | ||||||
| 3 | 1 |
gal | 1 |
Btu | |||||||||||
| 4 | |||||||||||||||
| 5 | g/mL density methanol changed to lb/gallon |
||||||||||||||
| 6 | 32 |
gallon | 506 |
Btu | 262 |
calories | 0.7918 |
g | 3785 |
mL | 1 |
lb | = |
??? |
calories |
| 7 | 1 |
lb | 1 |
Btu | 1 |
mL | 1 |
gallon | 454 |
g | |||||
| 8 | |||||||||||||||
| 9 | g/mL density changed to grams/gallon |
||||||||||||||
| 10 | 32 |
gallon | 1100 |
kJ | 1 |
calories | 0.7918 |
g | 3785 |
mL | = |
??? |
calories | ||
| 11 | 1 |
kg | 4.18 |
Joules | 1 |
mL | 1 |
gallon | |||||||
| 12 | |||||||||||||||
| 13 | g/mL density changed to grams/gallon |
molar mass g> moles |
|||||||||||||
| 14 | 32 |
gallon | 35278 |
kJ | 1 |
calories | 0.7918 |
g | 3785 |
mL | 1 |
mole |
= |
??? | calories |
1 |
kmol | 4.18 |
Joules | 1 |
mL | 1 |
gallon | 32.04 |
g | ||||||
(Round the below answers to 2 significant figures)
Problem 47: What is the calories in H2?
Problem 48: What is the calories in P6?
Problem 49: What is the calories in N10?
Problem 50: What is the calories in P14?
Problem 51: What is does Btu mean?
Problem 52: What formula goes in P6?
Problem 53: What cell canceled the "k" (kilo) in D10?
Problem 54: I found one value for heat of condensation that actually used calories. It was 270 calories per gram. How many calories are released from the condensation of 32 gallons of methanol using that value?

A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M | |
| 1 | calories released as methanol condenses |
Specific heat of water inverted |
amount water |
gallon to mL converion |
1mL H2O weighs 1 gram |
temp rise | |||||||
| 2 | 26000000 |
calories | 1 |
°C·g | ? |
? |
1 |
mL | = | 124 | °C | ||
| 3 | 1.0 |
calories | 55 |
gallons | ? |
? |
1 |
g | |||||
Problem 56: What goes in G3 and H3?

Problem 57: In Google, I did a search for "water heat of vaporization". Instead of the word "heat" what other word could I have used?

A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
|
| 1 | calories released as methanol condenses |
Water's Heat of vaporization inverted |
cal>J |
H2O molar mass to go from moles to grams |
1mL H2O weighs 1 gram |
mL to gallons |
gallons evaporated |
||||||||
| 2 | 26000000 |
calories | 1 |
mole | ? |
joule | ? |
? |
1 |
mL | ? |
gallon | = | ??? | gallons |
| 3 | 43500 |
Joules | 1 |
calorie | ? |
? |
1 |
g | ? |
mL | |||||
Problem 59: What goes in E2?
Problem 60: To eliminate moles in D2, we need to change moles of water into grams. What values go into G2 and H2?
Problem 61: What values go into G3 and H3?
Problem 62: What goes in K2?
Problem 63: What goes in K3?
Problem 64: How many gallons will get evaporated (N2)? (Hint: gallons is between 5 and 20)
There weren't that many gallons that would need to evaporate, so that's the design they went with. They were smart to let me do the chemistry calculations to see if what their design would work on paper. If it didn't work, they needed some recommendations. So learning these kind of calculations may be difficult but not as difficult as spending thousands of dollars and many days building an apparatus that isn't going to work.


Bonus 2: What is the formula for magnesium silicate?
Bonus 3: Magnesium silicate attracts these contaminants in the biodiesel: glycerin, sodium hydroxide, soap, and fatty acids. Why does magnesium silicate attract these?

Bonus 4: Attach an image of a magnetic stirrer for extra credit or describe why it is used in chemistry labs.

At the Veggie Van website, they list 10 things people can do to make us more energy independent.
Bonus 5: What are those 10 suggestions? Here's the Web page: http://www.veggievan.org/schools/

Instead of animal fat or vegetable oil as the feedstock for biodiesel, oils from algae shows a lot of promise. ASU has a department that specializes in algae research. Below is a link to that department's promotional brochure. It's a pdf file. There's only 2 pages of reading, but it's a little difficult to zoom in and pan around to read it.
http://larb.asu.edu/files/biofuel_brochure.pdf
Bonus 6: Read the brochure and report back on what 2 main benefits algae has over rooted plants.

Bonus 6: There's one person selling a book on making algae biodiesel at home and produced a video to promote it. Even though I'm all for alternative fuels, I'm a little skeptical of his claims. But it's still interesting. What are some of the things it says the book will teach you. Below is the link to the video.
http://www.youtube.com/watch?v=7Nf3M68S3ec
