Study Guide for the On-Campus Final for CHM151
Instructor: Ken
Costello
The emphasis
of this exam covers two areas:
|
Survival skills for learning chemistry |
|
Chemistry in a New Light |
||
|
|
Overcoming misunderstood Words and Symbols |
|
|
Building
Blocks |
|
|
Overcoming a lack of reality |
|
|
Force/Energy |
|
|
Overcoming too steep of a learning curve |
|
|
Mathematics |
Survival skills for learning chemistry
Overcoming
misunderstood Words and Symbols
Like I forewarned, there was going to be a lot of new words and symbols in this
course.
The metric system has own words and symbols.
There are several more, but the ones below are commonly used in
chemistry. Commit these to memory.
|
Metric prefix |
mega |
kilo |
deci |
centi |
milli |
micro |
nano |
pico |
|
English |
million |
thousand |
tenth |
hundredth |
thousandth |
millionth |
billionth |
trillionth |
|
Exponent |
106 |
103 |
10-1 |
10-2 |
10-3 |
10-6 |
10-9 |
10-12 |
|
Symbol |
M |
k |
d |
c |
m |
μ |
n |
p |
The words and symbols above are all about the same size. The sizes they represent are not even close to the same size. You must have reality of the sizes of the below lengths and be able to draw their approximate sizes.
![]() 4 inches
4 inches
![]() 1
decimeter (10 cm)
1
decimeter (10 cm)
![]()
![]() 1 inch
1 inch
2.5 centimeters
![]() 1 centimeter
1 centimeter
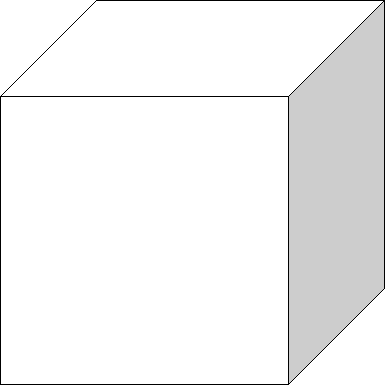
![]() 1/16 inch
1/16 inch
Chemistry deals with real volumes. You also
should be able to draw a cubic inch, cubic
centimeter (mL), and a cubic
decimeter (a liter) or a multiple of
any of these.
![]() 1 millimeter
1 millimeter
cubic decimeter 1 liter 1000 milliliters BUILDING
BLOCKS
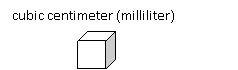

![]()

Chemistry is very much about building blocks. The smallest building blocks that have been proposed but not yet proven are strings of energy. These strings are very small and require 13 dimensions, not just 3. The image on the right is a representation of these strings sitting at the very smallest points of space. The extra dimensions sort of wrap upon themselves. The new Large Hadron Collider particle accelerator may actually give some proof to this theory this coming year. The collider just got back up and running last week. They will look for particles that simply pop out of existence as they leave our 3 dimensions and go into one of the other 10 dimensions. That will be very exciting news.
These points in space are 1.6 x 10-35 meters apart. In math you can take 1.6x10-35m and divide by 100 to get 1.6x10-37m, but in chemistry that presents a problem because there’s no way to ever see it using light. Light at a small enough wavelength (high enough frequency) to see these points will have so much energy it would create a black hole and disappear. That can be calculated with the formula for light energy, E = hv where v is frequency and h is Planck’s constant. By the way, the distance of 1.6x10-35 is called Planck’s length.
Below is a table of the building blocks for chemistry (On
the test I will replace red words with blanks that you fill in).
|
|
|
Strings of Energy |
|
|
||
|
|
|
Protons |
Neutrons |
Electrons |
|
|
|
Element: Contains atoms with same # protons |
|
Atom |
Ion: (atom with +/- charge) |
|
||
|
Compounds: (2 or more different atoms with ionic or covalent bonds) Examples: H2O, NaCl, CH4 |
Molecule: (2 or more atoms that can be same or different): Examples: O3, H2O, NaCl, CH4 |
Polyatomic ions: (2 or more different atoms with net +/- charge) Examples: SO42-, NH4+, NO3- |
|
|||
|
Macromolecule: (chains of smaller molecules) Examples: starch, cellulose, protein, DNA, polymers |
Ionic crystals: (stacks of + & - ions) Examples: NaCl, CaF2, MgO, K2CO3 |
Network solids: (stacks of non-metal atoms covalently bonded) Examples: diamond, SiC, quartz=SiO2 |
Molecular solid: stacks of small molecules) Examples Ice, dry ice, sugar, Aspirin |
|||
Force
and Energy
The main forces in chemistry
are electromagnetic forces, which include electrical, magnetic, and light
forces. The atom exists because of
electrical attraction between the protons in the nucleus and the electrons around it. Molecules exist because atoms have electrical
attraction to neighboring atoms. The
building blocks above are all created through electrical attraction and
repulsion. Chemical reactions are almost
entirely based on electrical attraction and repulsion. For example, to
understand the body, understand how chemicals in the body either attract or
repel each other. The situation where carbon monoxide is poisonous because it
has a stronger electrical attraction to red blood cells than oxygen does. Also, muscle contraction is dependent upon
the electrical attraction and repulsion forces of calcium (Ca2+),
sodium (Na+), and potassium (K+).
Below is a table that describes the
Electromagnetic forces that relate to chemistry.
|
Electromagnetic Forces |
||
|
Electrical |
Magnetic |
Light |
|
Repulsion
of like charges (proton/proton,
electron/electron, ions with like charges) Examples: ionic crystals, protein
shapes, VSEPR=molecule shapes, electron
orbital shapes, air pressure. Attraction
of unlike charges (protons/electrons, + & - ions) Examples: all bonds, ionic crystals,
protein shapes, nearly all chemical reactions, London Dispersion forces |
Magnetism pushes on any moving charge such as
electrons, protons, and all ions. Examples: Instruments based on
magnetism: mass spectrometer, NMR,
& MRI. Magnetism affects electron orbital shapes.
Unpaired electrons in atoms make materials magnetic. |
Radio waves move electrons (antenna) and moving
electrons create radio waves
(transmitter). High frequency radio
waves vibrate molecules (microwave oven).
Infrared light stretches bonds and vibrates molecules (heat lamp). Visible light pushes
electrons to higher orbitals. This
gives items color and allows photosynthesis .
UV light breaks bonds (sunburn). |
On the test I will leave out the words in red. Also, I will ask what does NMR and MRI stand
for.
Survival Skill of Overcoming a lack
of reality. The above table is all words. Let’s give it some reality.
Clap your hands. Technically your hands
never touch. The repulsion of electrons
in the proteins in your skin repelled each other, keeping the surface of the hands from touching. What you felt was electrical repulsion and
not the hands themselves. This
repulsion also pushed on the electrons in the air molecules between your hands,
which squeezes the air molecules together. The outer electrons of the
compressed air molecules create a chain reaction of repulsion on the electrons
of other air molecules. This is how
sound gets to your ears. Sound, voice,
and music are all the result of electrons repelling electrons.
cubic
inch
What allows
the chair you are sitting on to hold you up?
The electrons in the chair’s surface are repelling the fabric on the
seat of your pants. Also, the strength
of the chair is the attraction of protons and electrons between atoms. In other words the bonds between atoms give
it strength.
Electrons absorbing light give us vision. Find something blue or green to look at. Now learn the chemistry happening in your eye. A modified form of Vitamin A gets attached to
a protein called Rhodopsin. See image
below. When blue-green light
(represented by hv) hits the pi bond in the double bond
shown, it breaks the pi bond momentarily allowing the right end of the molecule
to swing around. The pi bond reforms but
now the right end has rotated. This new molecule sets off a signal and allows
you to see blue-green colors.
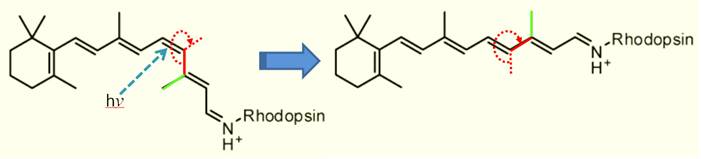
On the test I will show the molecule before
light hits it. You will draw the final
molecule (right side).
Force and energy go together because whenever a force causes
a movement that requires or releases energy.
Let’s look at how these various forms of energy relate to chemistry.
|
ENERGY |
||||
|
Mechanical |
Potential |
Kinetic/Heat |
Heat of Reaction |
Light |
|
Force
x distance= work energy. Pressure (force) of gas times distance it
expands (volume change) is work energy. |
For objects, more potential energy means higher above the ground. For
atoms, it means separating charges more, i.e., electrons or negative ions are
moved farther away from protons or positive
ions. |
Kinetic energy is the energy from movement. The kinetic energy of a collection of
atoms or molecules is its heat energy (enthalpy). Heat Capacity is heat energy per gram, lb., or mole. Specific Heat is
Heat Capacity per °C or °F. When substances change from solid to liquid to
gas, the atoms or molecules change speed, so heat energy changes. Energy from these phase changes are called Heat of Fusion (liquid>solid) and Heat
of Vaporization (liquid>gas). |
As elements combine to make compounds, energy is released, which is
called Heat of Formation. As compounds react, energy may be
released (exothermic) or absorbed (endothermic).
That’s called Heat of Reaction. |
Energy
of light is based on its frequency (or wavelength). The formula is E=hv. Where “v” is frequency and h is Planck’s constant. |
|
Electrical |
||||
|
Electrical power is watts. Watts times seconds
gives us energy in joules. |
||||
On the test I will
substitute the words in red above with blanks. You
fill in the missing word.
Mathematics
My biggest advice for mathematics is to learn dimensional analysis and do it using spreadsheets. In upper levels of chemistry, you will be required to use spreadsheets, but there’s no need not to take advantage of this technique now. The below problems are worked out. You will just need to decide where the units go. On the final exam, the units (dimensions) or values that are red will be replaced with blanks that you fill in. You don’t need to memorize what they are. Just figure out what needs to be there in order to give the answer the correct units.
|
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
|
1 |
Starting grams given |
NaOH Molar mass g>moles |
Ratio from balanced equation |
Turn moles Na2SO4 to grams |
|
Grams asked for |
|||||
|
2 |
5.00 |
grams
NaOH |
1 |
mole
NaOH |
1 |
mole Na2SO4 |
142 |
grams Na2SO4 |
= |
8.88 |
grams Na2SO4 |
|
3 |
|
|
40.00 |
grams NaOH |
2 |
moles NaOH |
1 |
mole
Na2SO4 |
|
|
|
|
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
|
1 |
Concentration in g/100mL times its mL gives
grams of HNO3 |
Ending 5% w/v inverted |
|
|
Final volume |
|||||
|
2 |
70.0 |
g HNO3 |
200 |
mL solution |
100 |
mL |
0.001 |
= |
2.80 |
Liters |
|
3 |
100 |
mL solution |
|
|
5 |
g
HNO3 |
milli |
|
|
|
|
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
|
1 |
Moles/Liter
times Liters gives moles of HNO3 |
molar
mass HNO3 |
Ending
5% w/v inverted |
|
|
Final volume |
|||||||
|
2 |
15.7 |
moles |
200 |
mL |
0.001 |
63.01 |
grams |
100 |
mL |
0.001 |
= |
3.97 |
Liters |
|
3 |
1 |
L |
|
|
milli |
1 |
mole |
5 |
g HNO3 |
milli |
|
|
|
|
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
|
1 |
5g/100mL
times 473mL gives grams |
Density
of pure acetic acid is 1.049g/mL |
pure
acetic acid |
||||||
|
2 |
5 |
g |
473 |
mL |
1 |
mL |
= |
22.5 |
mL |
|
3 |
100 |
mL |
|
|
1.049 |
g |
|
|
|
|
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
|
1 |
Liters
times moles per liter gives moles
NaOH |
moles of Aspirin |
Molar mass mol>g |
|
moles
per liter |
||||||
|
2 |
0.01692 |
L NaOH |
0.1026 |
mole
NaOH |
1 |
mole
Aspirin |
180.157 |
grams
Aspirin |
= |
0.3124 |
grams
Aspirin |
|
3 |
|
|
1 |
L
NaOH |
1 |
mole
NaOH |
1 |
mole
Aspirin |
|
|
|
|
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
P |
Q |
|
|
n |
R |
T |
/V |
|
= P |
|||||||||||
|
1 |
pounds > grams |
grams > moles (n) |
R constant |
Temp in Kelvin |
Divide by volume |
Atm > psi |
pressure of CO2 |
||||||||||
|
2 |
1/4 |
lb |
454 |
g |
1 |
mole |
0.0821 |
atm·L |
303 |
K |
|
|
14.7 |
psi |
= |
475 |
psi |
|
3 |
|
|
1 |
lb |
44.0 |
g CO2 |
|
mole·K |
|
|
2.0 |
Liters |
1 |
atm |
|
|
|
|
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
P |
Q |
R |
S |
T |
U |
V |
W |
|
1 |
P |
V |
/R |
/K
|
moles
> g |
wt. of air |
|
add
wt of tank |
Final
wt. |
||||||||||||||
|
2 |
3000 |
psi |
1 |
atm |
16.0 |
Liter |
|
mole·K |
|
|
28.8 |
g |
= |
??? |
g |
+ |
10.0 |
lb |
454 |
g |
= |
??? |
g |
|
3 |
|
|
14.7 |
psi |
|
|
0.0821 |
atm·L |
=(77-32)*5/9+273 |
K |
|
mol |
|
|
|
|
|
|
1 |
lb |
|
|
|
|
4 |
|
|
|
|
|
|
|
|
This
converts °F to K |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Electronic Configuration of
potassium
|
n=1 |
|
n=2 |
|
n=3 |
|
n=4 |
|
||||||||||||||||||
|
l=0 |
|
l=0 |
|
l=1 |
|
l=0 |
|
l=1 |
|
l=0 |
|
||||||||||||||
|
1s2 |
|
2s2 |
|
2p6 |
|
3s2 |
|
3p6 |
|
4s2 |
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
m=0 |
|
m=0 |
|
m=-1 |
m=0 |
m=+1 |
|
m=0 |
|
m=-1 |
m=0 |
m=+1 |
|
m=0 |
|
||||||||||
|
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
|
1 |
|
Mass
of water |
Degrees
cooled |
Heat
capacity of water |
|
Energy
|
||||
|
2 |
Energy lost to cool water to 0°C |
540 |
g |
22.0 |
°C |
4.18 |
J |
= |
Joules |
|
|
3 |
|
|
|
|
|
|
g·°C |
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|
|
5 |
Energy lost as water becomes ice |
Mass
of water |
Convert
g to moles |
Heat
of fusion of water |
|
Energy
|
||||
|
6 |
|
540 |
g |
1 |
mole |
6020 |
J |
= |
Joules |
|
|
7 |
|
|
|
18 |
g |
|
mole |
|
|
|
|
8 |
|
|
|
|
|
|
|
|
|
|
|
9 |
Energy lost as ice cools to -5°C |
Mass
of water |
Degrees
cooled |
Heat
capacity of ice |
|
Energy
|
||||
|
10 |
|
540 |
g |
5.0 |
°C |
4.18 |
J |
= |
Joules |
|
|
12 |
|
|
|
|
|
|
g·°C |
|
|
|
|
13 |
|
|
|
|
|
|
Total Joules |
= |
Joules |
|
Symbols

I said you would have to become a
symbologist if you want to learn chemistry.
At this point in chemistry, you won’t be skilled with all of the
materials that you covered, but you should be able to spot most symbols and
know generally where they belong. Below
are symbols and words that belong to the same category. On the right is the category they belong
to. On the
exam I will have them in a different order, but you will match them to the
correct one on the right.

On the test
I will leave off the labels of “Charge”, “Number of Protons”, etc. You will write them in.
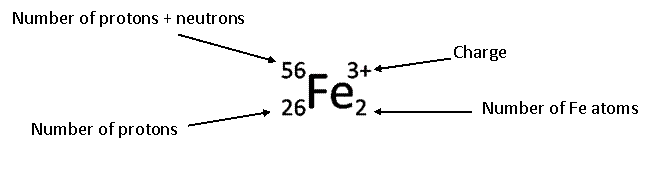
Below is an image used at the
beginning of the semester to illustrate how chemistry uses a bunch of
symbols. On the final I will ask you to
pick 7 of them and say what they stand for.
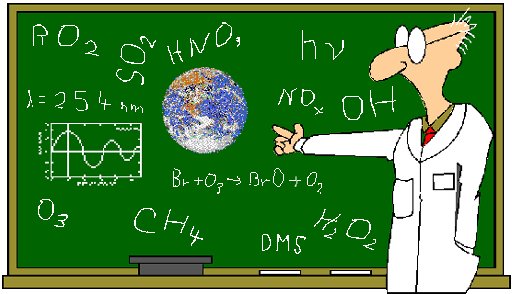
CHEMISTRY WORDS OF WISDOM
After many years of working with science
and chemistry, I came up with these 3 words of wisdom statements. Give an example of each of these.
1) Nothing is as complex as it looks or
as simple as it looks.
2) The difference between trash and
treasure is just the arrangement of the atoms.
3) The
difference between health and sickness is just the arrangement of the atoms.
(I give more explanation of these in my oral exam study guide for my CHM130 students. If you want to read that, here is the URL to that section:
http://www.chemistryland.com/CHM130W/18-Final/OralExam/OralExamFall10.htm#wisdom
Don’t use my examples given in that study guide as your examples. You can think of your own.
As an extra credit problem, I will
list the three Pitfalls of Learning. I
will see if you can match those to their symptoms. Review the first tutorial of the semester for that information.
That’s all.
Good luck on the online exam and this on
campus exam.
Mr. C.