In all versions of the textbook, read the first half of the chapter, which is section called: Hybridization and the Localized Electron Model.
The chapter also has a section called "The Molecular Orbital Model"; however, we are not covering that in this course. It's not on the competencies for CHM151. If you take an Organic Chemistry class, that's usually where the molecular orbital model is taught.
The problems were taken from the 8th edition. You don't need that textbook because I've reproduced the problems below.

Problem 1a: (Hint: To answer question (a) remember that a single line is always a sigma (σ) bond meaning the electrons are pointing at each other as they overlap. All double-bonds have one sigma bond. All double-bonds also have one pi bond.)
Problem 1b: (Hint: To identify which hybrid for carbon atoms, use the chart below. It makes sense because each hybrid creates, 4, 3, or 2 equivalent electrons.)
Number of atoms carbon is connected to |
Hybrid used |
4 |
sp3 |
3 |
sp2 |
2 |
sp |
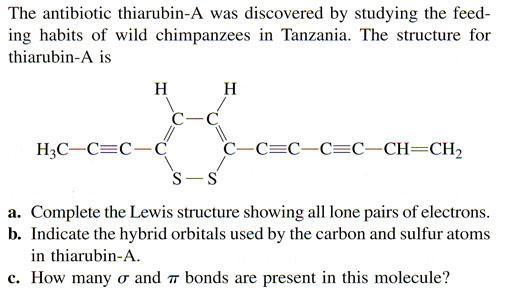
Problem 2b: Indicate the hybrid orbitals used by the carbon and sulfur atoms in thiarubin-A? (Use the table above to determine what hybrid orbitals are used by the carbon atoms. You don't have to be specific with which carbon atom uses what orbital, just which carbon hybrid orbitals were used in the molecule. For sulfur, you count the 2 lone pairs as if they were 2 more bonds. In other words, the 2 bonds and the 2 lone pairs have to spread out as much as possible. What hybrid orbital does that?).
Problem 2c: How many σ and π bonds are present in this molecule. (This is like Problem 1. For triple bonds, remember one of those bonds is a sigma bond and the other 2 are pi bonds.)
Problem 3b: How many carbon atoms are sp2 hybridized?
Problem 3c: Which atom is sp hybridized? (This one is tricky. If you roll cursor over the image, you will see the lone pair electrons. If you add the number of atoms connected plus the lone pairs on the central atom, that shows you what hybrid orbital is needed. If that adds up to 4, then the atom goes with sp3 orbital. If that adds up to 3, then it uses sp2. If that adds up to 2, then it is sp. For example, the top oxygen has 2 lone pairs and is connected to one other atom. That adds up to 3, so oxygen is using sp2 hybrid. The bottom nitrogen is the same (2 lone pairs + 1 connected atom) and is therefore sp2. The one with sp hybrid is either: (connected to 2 atoms and has no lone pairs) or (connected to one atom and has one lone pair).
Problem 3d: Do the same as in previous problems.
Problem 3e: Do the same as in previous problems.
Problem 3f: What is the N=N=N bond angle? If you answer 3c correctly, this one will be answered.
Problem 3g: (Hint: Oxygen has 2 lone pairs and 2 atoms connected. That adds up to 4. So sp3 creates 4 equal orbitals (a tetrahedron). What angle do tetrahedrons have? Note: In the free program called ChemSketch, it will give an even more accurate angle, but the tetrahedral angle is close enough)
Problem 3h: (Hint: I've already answered that in problem 3g. The textbook should have put this question before 3g, since you have to know the hybridization in order to know the angle.)