Synthesis (Combination)
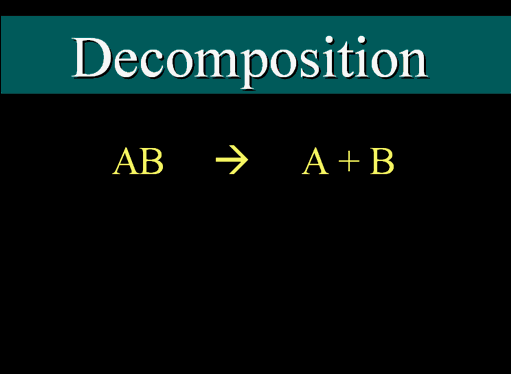
Decomposition
Single Replacement/Single Displacement
Double Replacement/Double Displacement
Oxidation (Combustion)
1a) 2KClO3 --> 2KCl + 3O2
1b) MgSO4·7H2O --> MgSO4 + 7H2O
1c) N2 + 3H2 --> 2NH3
1d) C3H8 + O2 --> 3CO2 + 4H2O
1e) AgNO3 + NaCl --> AgCl + NaNO3
1f) 3C + Fe2O3 --> 2Fe + 3CO
1g) NH4I + Cl2 --> NH4Cl + I2
1h) C3H5(NO3)3 --> CO2+H2O+N2+O2
1i) Al + H2SO4 --> Al2(SO4)3 + H2
1j) H2SO4 + 2NaOH --> 2HOH + Na2SO4
Problem 1 (Types of reactions):
Look at the chemical reactions on the left. Identify what type of reaction is taking place (pick from list above).
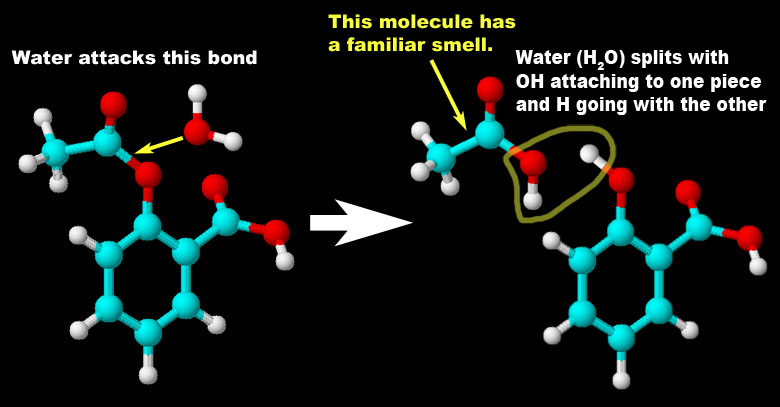
Problem 3 (more decomposition): Chemistry uses different ways to represent a chemical reaction. The AB -> A + B is a general way to express a decomposition reaction. Roll cursor over the image on the left to see an animation of a specific decomposition reaction. Write the chemical equation that matches this reaction. (If animation doesn't play right, you can try to click this link to see it in Flash format. Use back arrow to get back to quiz).
4a) How many carbon atoms does propyl alcohol have?
4b) How many oxygen atoms does acetic acid have?
4c) How many hydrogen atoms does the pear flavor have? (Note: its chemical name is propyl acetate)


1) CO2 + H2O --> H2CO3
2) H2CO3 --> H+ + HCO3-
Problem 5: Respiratory acidosis occurs when a person is not breathing out carbon dioxide fast enough (hypoventilation). This can be caused by breathing problems such as pneumothorax (collapsed lung), asthma (restricted airways), emphysema (nonelastic air sacs), pneumonia (mucus in lungs), or sedatives (slowed breathing). Carbon dioxide in the blood will produce acidic hydrogen ions (H+) in a two step process. That causes the blood to be more acidic than normal (acidosis). That's also called acidemia (acidic blood).
5a) What is the name of H2CO3?
5b) What is the name of the HCO3- ion?
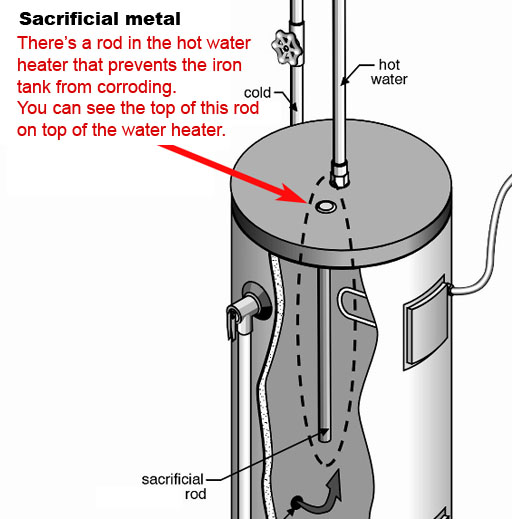
Problem: 6: Single replacement and sacrificial metal:
Most hot water heater tanks are made from steel (iron), which can corrode in the presence of oxygen and water. Here is the reaction.
2Fe + O2 + H2O --> 2Fe(OH)2
Each iron atom loses two electrons, which forms iron (II) hydroxide.
A more reactive metal, such as magnesium (or zinc) can sacrifice itself by giving iron its electrons and forming magnesium hydroxide (magnesium corrodes instead of iron). So the single replacement reaction looks like this:
Fe(OH)2 + Mg --> Mg(OH)2 + Fe
If the sacrificial rod is doing its job, you may see the color of Mg(OH)2. If not, the iron case of the heater is decomposing and you will see Fe(OH)2.
6a) What color is Fe(OH)2?
6b) What color is Mg(OH)2?


Problem 7: Combustion
reaction (a type of oxidation):
On cold mornings, you often see vapor coming from car exhausts. Even on warmer days, if you hold a beaker near the exhaust of a car whose engine has not warmed up, you can see condensation (bottom image). The combustion of gasoline produces (like all hydrocarbons) carbon dioxide and water. The average length of gasoline molecules is about 8 carbons long. So lets use octane to represent gasoline.
2C8H18 + 25O2 --> 16CO2 + 18H2O
Most people assume this vapor cloud is water vapor and the drops of liquid as just water; however, it's not just water. It's seltzer (carbonated) water. They forget that carbon dioxide is present and there has been a synthesis reaction.
CO2 + H2O --> ?
7a) Complete the above synthesis reaction.
7b) There's a small amount of sulfur in gasoline with some getting converted to SO3. It combines with water to form an acid. Complete this synthesis reaction:
SO3 + H2O -> ?
7c) What is the name of the acid that gets produced?