
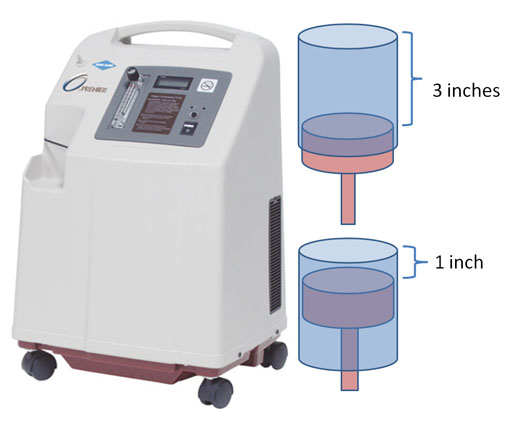
Problem 1: What is the molar mass of NO2?
Problem 2: Will it sink or rise in the air?

3a) Air weighs 28.8 grams per mole. How many grams per mole does chlorine gas (Cl2) weigh?
3b) Will chlorine gas stay close to the ground or rise up into the air?
3c) How many grams is 300,000 lbs mentioned above, knowing that 1 lb = 454 grams.
3d) How many moles of Cl2 is 300,000 lbs of Cl2? (use grams from 3c).

Problem 4: What is the correct way of explaining how a vacuum cleaner works? (A or B?)
A) The spinning
motor creates a partial vacuum which creates a suction effect and draws
the dirt into the vacuum cleaner.
B) The spinning motor pushes air out of the vacuum cleaner reducing
the number of collisions of air inside. The outside air molecules then
collide more often which causes the air to rush in knocking dirt in
as it goes.


A few years ago a sad accident happened to a couple of college students. They learned about PV=nRT and wanted to see the pressure that dry ice would create in this pressure cooker. Unfortunately, the safety pressure release valve didn't work because the dry ice had created ice on the valve which blocked it. The pressure cooker exploded and killed both students. Again, we have to respect the pressure built up with liquids (or solids) turn into gases.
Problem
7: If a pound of dry ice was used and the pressure cooker has a volume of 6 quarts, what pressure in PSI could have occurred when all the dry ice turned to CO2 gas and reached a temperature of 70°F (cell P3)?
Like in the tutorial, if pressure is asked for, solve the PV=nRT formula for pressure, which you can see is P=nRT/V. This problem gets a bit more complicated because of the English measurement units need to be converted to metric units. See below table for setup help.
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
P |
Q |
R |
S |
|
| 1 | n |
R |
T |
/V |
=P |
||||||||||||||
| 2 | pounds > grams |
grams > moles (n) |
R constant |
70°F > Kelvin |
Divide by volume |
Atm > psi |
pressure of CO2 |
||||||||||||
| 3 | 1 |
lb | 454 |
g | 1 |
mole | 0.0821 |
atm·L | =(70-32)*5/9+273 |
K | 1 |
qt | 14.7 |
psi | = |
??? |
psi | ||
| 4 | 1 |
lb | 44.0 |
g CO2 | mole·K | 6.0 |
qt | 0.946 |
Liters | 1 |
atm | ||||||||
Problem
8: What units cancel in the above setup?
Problem
9: What unit or units remain?
Problem
10: What formula goes into R3?

The results of a basketball game was called into question with allegations of the basketball being filled with a gas other than air. Your task is to investigate. The ideal gas law PV=nRT may help with some rearranging. Divide both sides by n gives PV/n=RT, now divide both sides by PV to get 1/n=RT/PV. Now multiply by both sides by grams to get g/n=gRT/PV.
"g/n" is grams per mole and that is what the Periodic Table lists for each element
(atomic mass). So the final formula would read:
Atomic wt=gRT/PV
The standard radius of basketballs are 12.4 cm. Using formula for sphere V= 4/3 x pi x r3, you can calculate its volume. The pressure
is 29.4 psi and the temperature is 81°F (27°C,300K) You weigh
the basketball and it weighs 654.31 g. You let out the gas and find the
empty weight as 600.00g.
(Hint: turn 29.4 psi to Atm (14.7psi=1atm), convert
cm3 to liters, use Kelvin, and use 0.0821 for R).
Calculate gRT/PV and match it to the element that has that
atomic mass. The below spreadsheet helps with the layout
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
P |
Q |
|
| 1 | g |
R |
T |
/V |
/P |
= Atomic wt |
|||||||||||
| 2 | 654.31-600.00 g |
R constant |
Temp in Kelvin |
Ball volume in cm3 |
cm3 > L | Divide by volume |
psi > atm | g/mole=atomic wt |
|||||||||
| 3 | 54.31 |
g | 0.0821 |
atm·L | 300. |
K | 1000 |
cm3 |
14.7 |
psi | = |
??? |
g | ||||
| 4 | mole·K | =4/3*pi()*(12.4)^3 |
cm3 | 1 |
L |
29.4 |
psi | 1 |
atm | mole | |||||||
Problem 12: What cell cancels out the unit of "atm" in N4?
Problem 13: What is the g/mole in P3? (Note: That's the same as atomic wt)
Problem 14: What gas was used to fill the ball?
Problem 15: What formula is used in P3?
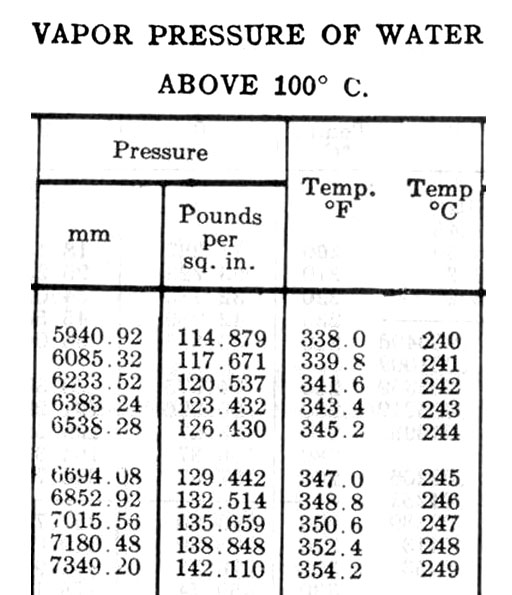

With gas problems, you always start with PV=nRT. There are no gallons in this formula, but there are moles (n), and we know we can turn moles of water to grams then grams to liters. So we rearrange PV=nRT to get PV/RT=n and go from there. See spreadsheet for layout help
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
P |
Q |
R |
S |
T |
U |
V |
W |
|
After this we have moles H2O (n) |
Now we have gallons of liquid water needed to fill a 1.25 Liter piston with water vapor per revolution. |
||||||||||||||||||||||
| 1 | P |
V |
/R |
/T |
Molar mass H2O |
Total gallons of water to supply 1.25L piston with water vapor for 1 hr at 80rev/min at 248°C
|
|||||||||||||||||
| 2 | From table at 248°C |
Piston vol. |
R constant inverted |
divide by temp 248°C>Kelvin |
moles> g |
g > Liter |
Liter > gal |
||||||||||||||||
| 3 | 138.848 |
psi | 1 |
atm | 1.25 |
L | mole·K | ??? |
g H2O | 1 |
Liter H2O | 1 |
gal | 80 |
revs |
60 |
min | = | ??? | gallons | |||
| 4 | 14.7 |
psi | 0.0821 |
atm·L | =248+273 |
K |
1 |
mole |
1000 |
g H2O | 3.785 |
L | 1 |
min | |||||||||
Problem 17: If the temperature was lowered to 240°C, which two cells would need to be changed? And what new value would go into A3?
Problem 18: If the steam engine ran at 75 revolutions per minute, what cell needs to be updated?
Problem 19: What is the formula that goes into V3? (As usual you can omit cells that have a "1" in them.)