For all editions read the sections labeled:
Acid-Base Titration
Neutralization Analysis
For all editions read the sections labeled:
Oxidation-Reduction Reactions
Balancing Oxidation-Reduction Reactions

A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
|
| 1 | a. | Desired liters x moles per liter of diluted solution=moles |
18M concentrated sulfuric acid inverted |
mL of conc.H2SO4 |
||||||||
| 2 | 1.00 |
Liter diluted | 0.50 |
moles sulfuric acid | 1 |
Liter of concentrated | milli |
= |
??? |
mL of concentrated | ||
| 3 | Liter diluted | 18 |
moles sulfuric acid | 0.001 |
||||||||
| 4 | ||||||||||||
| 5 | b. | Desired liters x moles per liter of diluted solution=moles |
12M concentrated HCl inverted |
mL of conc. HCl |
||||||||
| 6 | 1.00 |
Liter diluted | 0.50 |
moles hydrochloric acid | 1 |
Liter of concentrated | milli |
= |
??? |
mL of concentrated | ||
| 7 | Liter diluted | 12 |
moles hydrochloric acid | 0.001 |
||||||||
| 8 | ||||||||||||
| 9 | c. | Desired liters x moles per liter of diluted solution=moles |
1 mole NiCl2 makes 1 mole NiCl2·6H2O |
moles to grams |
grams NiCl2·6H2O |
|||||||
| 10 | 1.00 |
Liter diluted | 0.50 |
moles NiCl2 | 1 |
mole NiCl2·6H2O | 237.69 |
g NiCl2·6H2O |
= |
??? |
g of NiCl2·6H2O | |
| 11 | 1 |
Liter diluted | 1 |
mole NiCl2 | 1 |
mole NiCl2·6H2O |
||||||
| 12 | ||||||||||||
| 13 | d. | Desired liters x moles per liter of diluted solution=moles |
16M concentrated nitric acid inverted |
mL of conc.HNO3 |
||||||||
| 14 | 1.00 |
Liter diluted | 0.50 |
moles nitric acid | 1 |
Liter of concentrated | milli |
= |
??? |
mL of concentrated | ||
| 15 | 1 |
Liter diluted | 16 |
moles nitric acid | 0.001 |
|||||||
| 16 | ||||||||||||
| 17 | e. | Desired liters x moles per liter of diluted solution=moles |
moles sodium carbonate to grams |
grams of sodium carbonate |
||||||||
| 18 | 1.00 |
Liter diluted | 0.50 |
moles sodium carbonate | 105.99 |
grams Na2CO3 | = | ??? |
grams Na2CO3 | |||
| 19 | 1 |
Liter diluted | 1 |
mole Na2CO3 | ||||||||

A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
|
| 1 | mL times moles/liter equal millimoles |
adjust for # of H/OH | millimoles of either H+ or OH- |
|||||||||
| 2 | 50.0 |
mL | 0.100 |
moles Hydrochloric acid | 1 |
H+ | = |
??? |
millimoles H+ | |||
| 3 | 1 |
Liter of Hydrochloric acid solution | 1 |
molecule | ||||||||
| 4 | ||||||||||||
| 5 | 100.0 |
mL | 0.200 |
moles Nitric acid | 1 |
H+ | = | ??? |
millimoles H+ | |||
| 6 | 1 |
Liter of Nitric acid solution | 1 |
molecule | ||||||||
| 7 | ??? |
Total millimoles H+ | ||||||||||
| 8 | ||||||||||||
| 9 | 500.0 |
mL | 0.0100 |
moles Calcium hydroxide Ca(OH)2 | 2 |
OH- | = | ??? |
millimoles OH- | |||
| 10 | 1 |
Liter of calcium hydroxide solution | 1 |
molecule | ||||||||
| 11 | ||||||||||||
| 12 | 200.0 | mL | 0.100 |
moles Rubidium hydroxide RbOH | 1 |
OH- | = | ??? |
millimoles OH- | |||
| 13 | 1 |
Liter of Rubidium hydroxide solution | 1 |
molecule | ||||||||
| 14 | ??? |
Total millimoles OH- | moles/liter (M) of excess H+ or OH- |
|||||||||
| 15 | ??? |
mL | Total volume of all solutions | ??? |
millimole excess of H+ or OH- (H7-H14) | = |
??? |
moles | ||||
| 16 | =A15 | Divide by total mL from A15 | Liter | |||||||||
Problem 3: What formula goes into H9?
Problem 4: What formula goes into K15?

82a. SrCr2O7. For strontium dichromate first realize that strontium (Sr) is an alkaline earth metal (column 2) so it has a +2 oxidation number. The 7 oxygen atoms have -2 oxidation number each so that's -14 total. The oxidation number of the two chromium atoms have to make the -14 and +2 add up to zero. -14 and +2 add up to -12, so we need the two chromium atoms to have a total of +12. You can figure what just one chromium atom has.
82b. CuCl2. This one is easy. Chlorine is always -1. You can figure out what copper has to be.
82c. O2. Elements not bonded to a different element always has an oxidation number of 0.
82d. H2O2. This is easy to see with written the way they connect, which is HO-OH. Now we see that oxygen has a bond to another oxygen. That's when oxygen has an oxidation of -1 instead of -2. You know what the oxidation of hydrogen is.
82e. MgCO3 (magnesium carbonate). These oxygens are all bonded to the carbon atom so these 3 oxygen atoms are all -2 oxidation number each. Magnesium is an alkaline earth metal so it is always +2. So add up the 3 oxygen atoms and add it to the positive oxidation number of magnesium and you will find out what carbon has to be to make the total zero.
82f. Ag. Same rule as (c).
82g. PbSO3. This one is a little tough. Sulfur can have a negative or positive oxidation number (lose or gain electrons) depending on what it combines with. The tip here is that SO3 combined with a metal indicates the SO3 is a polyatomic ion, with 3 oxygen atoms attached to one sulfur atom. This is sulfite (SO3)2-, which has a negative 2 charge. If sulfite has a -2 charge, then lead (Pb) must have a +2 charge and therefore a +2 oxidatin number. These oxygen atoms have -2 each, so that's -6 for the 3 oxygen atoms. So if the oxygen atoms account for -6 and Pb accounts for +2, what must sulfur's oxidation be for the charge (and oxidation numbers) to add up to zero?
82h. PbO2. This one is easy. You know what oxygen normally is so what must the Pb be in this compound (It's not the same as Pb in the above problem).
82i. Na2C2O4. Note that these oxygen atoms are bonded to carbon and there's no oxygen to oxygen bonds. So these oxygen atoms are all -2 oxidation number. Sodium (Na) is an alkali metal so it's alway +1. So when you add these 4 oxygen atom's oxidation numbers to the two sodium atom's oxidation numbers you will get a number that carbon atoms must have to cancel.
82j. CO2. This one is easy. It's like (h).
82k. (NH4)2Ce(SO4)3. This one is big and ugly. It's good to recognize the polyatomic ions of NH4+ (ammonium) and SO42- (sulfate). NH4+ is a +1. Since hydrogen is +1 that totals +4 for the hydrogen atoms. What does nitrogen have to be to reduce the +4 down to +1? Sulfate is -2. Since each oxygen is -2, they account for -8. What does sulfur have to be if it can take -8 and turn it into -2? Cerium (Ce) is fairly easy. You have 2 NH4+ of +1 each (+2 total) and 3 sulfates at -2 each (-6 total). So what does Ce have to be in order for the +2 and the -6 to add up to zero?
82L. Cr2O3. This one is easy. Just note that all oxygen atoms attach to chromium and not to another oxygen. So all oxygen atoms are -2. So what does that force chromium (Cr) to be?
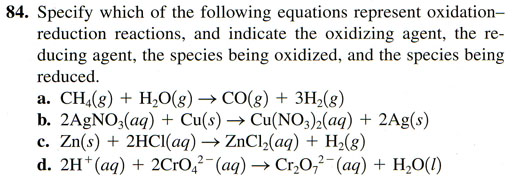
84a. CH4(g) + H2O(g)--> CO(g)+ 3H2(g)
This chemical reaction is a little unsual in the fact that water is being split and pure hydrogen is being formed. That happens when reacting with a reactive metal like pure sodium or potassium but not methane. This reaction only happens at very high temperatures. This reaction is used to create hydrogen gas that is then used to make ammonia (NH3) which is used to make fertilizer. Now on to the task for seeing if oxidation occurred (a loss of electrons) or reduction occurred (a gain of electrons which therefore reduced any positive charge).
CH4 [Here we have hydrogen which is +1 when combined. So 4 hydrogens total +4 meaning the carbon must be -4. ]
H2O [Hydrogen is still +1 and oxygen is -2 like normal.]
CO [Oxygen is -2 which means this carbon is +2. So we see that carbon started off as -4 but ended up +2. So it had to lose 6 electrons. . In other words the carbon atoms must have given electrons to other atom making that atom more negative (in other words it reduced its positive charge). That makes carbon the reducing agent and at the same time the species being oxidized (the one which lost electrons). So what atom did it give its electrons? Let's look at the final hydrogen.]
3H2 [Any element that is not combined with a different element is assigned a zero oxidation number. So hydrogen started off as +1 but ended up as 0. So each hydrogen atom received an electron from the carbon atom. Atoms that receive (or take) electrons are called oxidizers or oxidizing agent because oxygen is known for doing just that (taking electrons]. Being the oxidizing agent that also makes it the species being reduced.
84b. 2AgNO3(aq) + Cu(s) --> Cu(NO3)2(aq) + 2Ag(s)
AgNO3 [It helps to recognize that NO3 is the nitrate ion, which is -1. So that makes Ag +1. We don't have to find the oxidation number of nitrogen and oxygen because in the products the NO3- (nitrate ion) is still intact and therefore the same -1 charge. So no electrons got gained or lost regarding the nitrate ion.]
Cu(s) [A single element is zero oxidation number. It has no charge]
Cu(NO3)2 [Here we have two nitrate ions with -1 each or -2 total. So the copper must be +2. So we see that copper went from zero to +2. To accomplish that it must have lost 2 electrons. Again something that loses its electrons is like being attacked by oxygen. So it is being oxidized or is called the species being oxidized. So you can look at it as losing electrons (being oxidized) but also look at it as giving electrons to another atom and therefore reducing that atoms positive charge. So that makes the copper also the reducing agent.]
Ag(s) [Elemental silver like pure elements have zero oxidation number. So Ag started as +1 but ended up as zero. So what is the name given to Ag+ because it that took electrons from the copper? What is the name of this Ag+ because it reduced its charge down to zero?
84c. Zn(s) + 2HCl(aq) --> ZnCl2(aq) + H2(g) [This one is not too difficult. See if you can identify the oxidizing agent (same as species being reduced) and the reducing agent (same as the species being oxidized).
84d. 2H+(aq) + 2CrO42-(aq) --> Cr2O72-(aq) + H2O(l) [This one is bit difficult so I will give some hints on it.
H+ [This is obviously a +1 charge and therefore +1 oxidation number]
CrO42- [Chromate ion. The 4 oxygen atoms total -8. Since the whole CrO42- ion is -2 then Cr (chromium) must be +6.]
Cr2O72- [Dichromate ion. The 7 oxygen atoms total -14. Since the whole Cr2O72- ion is -2, then the two chromium atoms must total +12 in order to reduce -14 to -2. So each chromium atom must be +6. That means the chromium atom did not change its oxidation number and therefore did not gain or lose electrons.]
H2O [Hydrogen is +1 and oxygen is -2. So they didn't change their oxidation numbers either. This means the whole chemical equation (reaction) is not an oxidation-reduction reaction. One of the oxygen atoms (O2-) comes off of the first chromate ion and attached itself to the 2 H+ to make H2O. So no electrons transferred. There was just a rearrangement of atoms.]

A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
I |
M |
N |
O |
P |
Q |
R |
|
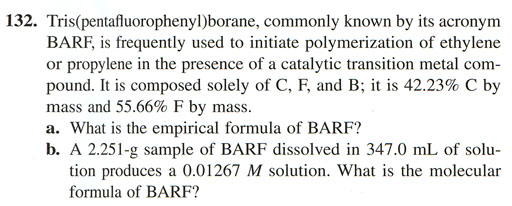
1 |
Analyzer readout (mass%) | Take % of 100g |
grams to moles |
moles in 100 g |
Make integers |
g/mol empirical formula | |||||||||||||
2 |
Carbon |
42.23% |
42.23 |
grams | 1 |
mole | = |
3.5192 |
moles C | ÷ |
0.1952 |
= |
18 |
12.00 |
g | = |
216.0 |
g | |
3 |
12.00 |
grams | 1 |
mole | |||||||||||||||
4 |
Fluorine |
55.66% |
55.66 |
grams | 1 |
mole | = |
2.9295 |
moles F | ÷ |
0.1952 |
= |
15 |
19.00 |
g | = |
285.0 |
g | |
5 |
19.00 |
grams | 1 |
mole | |||||||||||||||
6 |
Boron |
2.11% (to get 100%) |
2.11 |
grams | 1 |
mole | = |
0.1952 |
moles B | ÷ |
0.1952 |
= |
1 | 10.18 |
g | = |
10.18 |
g | |
7 |
10.81 |
grams | 1 |
mole | |||||||||||||||
8 |
write grams over mL to get g/mL | cancel m | Invert moles/liter to cancel L | Empirical formula = C?F?B? |
grams per mole | 511.2 |
g | ||||||||||||
9 |
2.251 |
gram sample | milli |
1 |
Liter | = | ??? |
grams | <-This is molar mass for C12 | ||||||||||
| 10 | 347.0 |
mL | 0.001 |
0.01267 |
moles | mole | |||||||||||||
| 11 | If ?=1, then empirical formula is the same as molecular formula | ||||||||||||||||||
12 |
Molar mass of compound from H9 |
=H9 |
g/mol | = |
? |
x | C?F?B? | = ? | |||||||||||
13 |
Mass of 1 mole of Empirical formula |
511.2 |
g/mol | Actual Molecular formula | |||||||||||||||