TEXTBOOK READINGS |
|
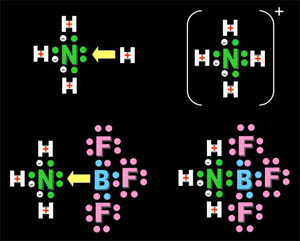
Atoms, Molecules, and Ions |
Author: Zumdahl
6th Edition:
pages 56-58
7th Edition:
pages 52-55
8th Edition:
pages 52-54 |
Author: Tro
2nd Edition:
pages 42-54 |
|
An Introduction to the Periodic Table |
Author: Zumdahl
6th Edition:
pages 58-61
7th Edition: pages 55-57
8th Edition: pages 54-57 |
Author: Tro
2nd Edition: pages 55-63 |
|
Naming Simple Compounds |
6th Edition:
pages 62-72
7th Edition:
pages 55-67
8th Edition:
pages 57-67 |
Author: Tro
2nd Edition: pages 84-97 in Chapter 3 |
|
Points of Interest |
Author: Zumdahl
Berzelius, Selenium, and Silicon
6th Edition:
page 50
7th Edition:
page 46
8th Edition:
page 46 |
Author: Tro
Chemistry in your day: Atoms and Humans
2nd Edition: page 49 |
PROBLEMS |
|
Problem 1: How would you find the number of chalk molecules (CaCO3) it takes to write your name on the board? Explain how you would do it and what calculations would you do. |
|
Problem 2: You may have noticed that when water boils, you can see bubbles that rise to the surface of the water. Whic of the following is inside these bubbles? Explain:
a. Air
b. Hydrogen and oxygen gas
c. Oxygen gas
d. Water vapor
e. Carbon dioxide gas |
|
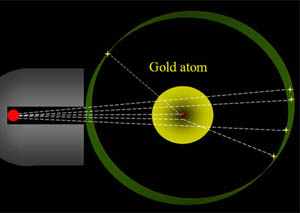
Problem 3: If atoms are about 99.99999999% empty space, then why does matter feel solid? |
|
Problem 4a: Why do we call Ba(NO3)2 barium nitrate, but we call Fe(NO3)2 iron (II) nitrate?
Problem 4b: Instructor's follow-up question: Is barium nitrate toxic? |
Problem 5: Complete the following table:
Notice that I wrote 53/26 to indicate that the 53 is superscript (53Fe2+), which means this iron's protons and neutrons add up to 53. 26 is a subscript (26Fe2+) meaning that iron contains 26 proton. You can do the same in your typed answers. Instead of submitting a table, just have your answer after the appropriate letter. For example, the first answer is "A=26". The second answer is "B=27". Answer all the way up to letter "L".
Symbol |
Number of Protons in Nucleus |
Number of Neutrons in Nucleus |
Number of Electrons |
Net Charge |
53/26Fe2+ |
A. |
B. |
C. |
D. |
E. |
26 |
33 |
F. |
3+ |
G. |
85 |
125 |
86 |
H. |
I. |
13 |
14 |
10 |
J. |
K. |
L. |
76 |
54 |
2- |
|
|
Problem 6: (This problem is not in textbook. It is created by the instructor.)
It's not a good idea to write down the combination to your combination lock, but you could hide that from people by using the element symbol which has the atomic number that matches the combination number. Use ion charge to indicate clockwise or counter clockwise. Below is the original instructions to open this lock.
Turn clockwise two turns to 30, one turn counterclockwise to 19 , one turn clockwise to 8. Find the elements that have the atomic number of these numbers. For example, zinc is atomic number 30. To indicate clockwise two turns show zinc as 2+ charge. So Zn2+ would be the first number. Find the other elements symbols that have atomic numbers that match the last two numbers. Use charge of "-" to indicate counterclockwise and "+" to indicate clockwise. Problem 6a: So what is the combination written with element symbols?
Problem 6b: A chemist would suspect something fishy with the last two symbols. Why?
|
Nomenclature problems |
Problem 7: For "a-d," write the name. For" e-h," write the formula.
|
a. Hg2O
b. FeBr3
c. CoS
d. TiCl4
e. tin (II) nitride
f. cobalt (III) iodide
g. mercury (II) oxide
h. chromium (VI) sulfide |
Additional Exercises |
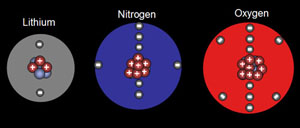
Problem 8: Atom and its subatomic particles
Which of the following statements is (are) true? For the false statements, correct them.
a. All particles in the nucleus of an atom are charged?
b. The atom is best described as a uniform sphere of matter in which electrons are embedded.
c. The mass of the nucleus is only a very small fraction of the mass of the entire atom.
d. The volume of the nucleus is only a very small fraction of the volume of the atom.
e. The number of neutrons in a neutral atom must equal the number of electrons. |
Problem 9: Systematic names:
The formulas and common names for several substance are given below. Give the systematic names for these substances. Follow-up questions: What are each of these substances used for? Explain why the common name may have been chosen for this substance?
| Common Name |
Formula |
Systematic name |
What is this used for? |
Explain why the common name was chosen |
| a. Sugar of lead |
Pb(C2H3O2)2 |
|
|
|
| b. Blue vitriol |
CuSO4 |
|
|
|
| c. Quicklime |
CaO |
|
|
|
| d. Epsom salts |
MgSO4 |
|
|
|
| e. Milk of magnesia |
Mg(OH)2 |
|
|
|
| f. Gypsum |
CaSO4 |
|
|
|
| g. Laughing gas |
N2O |
|
|
|
|
 |
Problem 10:
Give the systematic name of:
a: H2S (rotten egg smell)
b: SO2 (smell of burnt match)
c: SF6 (aerosol can propellant)
d. Na2SO3 (dried fruit preservative)
|