
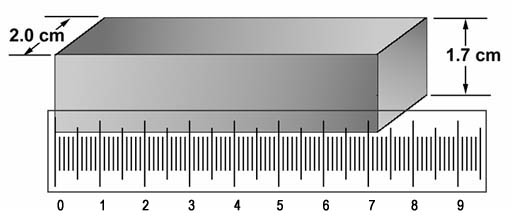
This bar was weighed and found to be 198 grams.
Problem 1: What is the density of this iron bar in grams per cubic centimeter?
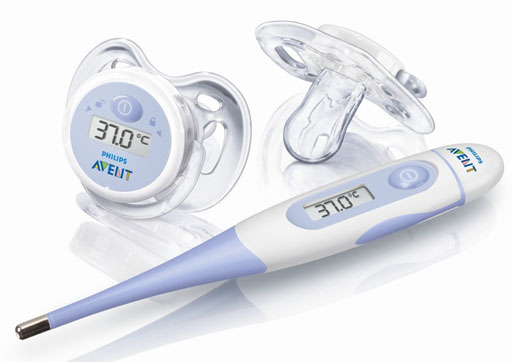
Here are some digital thermometers being advertised. Two are pacifiers. Problem 2: What is significant about the temperature being shown?

This is a Fahrenheit thermometer. Problem 3: What temperature would you say it is reading?

There are times that using a traditional thermometer is not practical. These infrared thermometers measure the infrared radiation coming from an object. To target the place where you are measuring, laser beam is used. The top/left image is a man checking the temperature of an exhaust pipe. The bottom/left image shows a person measuring molten metal.
Here is a link to one brand of infrared thermometers being sold at Amazon.
Infrared/non-contact thermometer link.
Problem 4: Scroll down and report what uses they show in their images.


Here is a butterfly being weighed. It shows 0.2735 grams. That's light. It would take about 18 of them to weigh the same as a nickel (5g). Most living things have a density about the same as water. Problem 6a: So if this butterfly has a density of water (1g/cm3), what is the volume (cm3) of this butterfly? (Hint: You don't need a calculator)
Problem 6b: Thirty drops of water is equivalent to 1 cm3. How many drops of water would have the same volume of our butterfly?.

Butterfly meets Aerogel. Aerogel has the lowest density of any solid. Some aerogels are made of silica (silicon dioxide like sand or quartz). This silica is like a sponge consisting of mostly air. The density of this aerogel is as low as 0.0011 grams per cubic centimeter. If a butterfly is similar to bees, then it can carry about half of its own weight. This cube of aerogel looks like about 10 cm on each side. The larger Monarch butterflies weigh about 0.80 grams. Problem 7: Can the larger butterfly carry this cube? If it can't carry it, how many butterflies would be needed (Hint: This can be calculated in your head).

(By the way I calculated that a 2,000 sq .ft. house that had walls made of aerogel is light enough for a person to lift the whole house.)
A |
B |
C |
D |
E |
F |
G |
H |
I |
|
| 1 | mass to lift |
lbs > grams |
invert density to get cm3 |
Volume of 50 lb aerogel |
|||||
| 2 | 50 |
lb |
454 |
g |
= |
??? |
cm3 |
||
| 3 | 1 |
lb |
|||||||
| 4 | |||||||||
| 5 | Take cube root of volume to get side |
||||||||
| 6 | =H2^0.333 |
= |
???? |
cm |
|||||
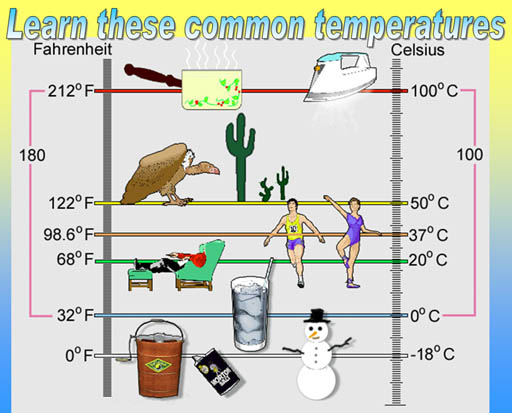
Problem 9: Why is there an ice cream maker, salt container, and snowman at the 0°F (-18°C) mark?
Problem 10: Convert these 6 temperatures to degrees Kelvin.