

| <-CHM151 Home Page | |
Quiz for Chemistry in a New Light |
|
| Copy each question below into an email and answer the questions there. (No need to copy the images). Mail your answers to your instructor at chm151@chemistryland.com | |
 |
1. You learned that chemistry involves 3 areas of focus: Building blocks, Force/Energy, and math. Let's say you are teaching your little girl how to make a cake. You will explain it to her using these 3 areas of focus. 1a: What building blocks will you mention that make a cake? 1b: What force or energy aspects will you mention? 1c: What math is involved in making a cake?
|
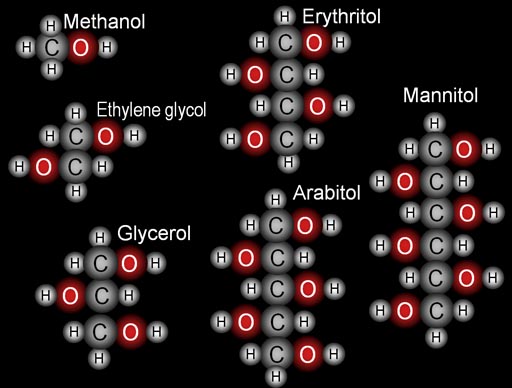 |
2. Building blocks: |
 |
3) The longest of the above molecules is mannitol. It has 6 carbon atoms. The molecule that would be the next in the series would be volemitol, which has 7 carbon atoms. It gets its name from a mushroom from which it was first isolated. What is the scientific name of that mushroom? (Hint: Go to Wikipedia and look up volemitol.) |
 |
4. Force and Energy: Here are three compounds from #2. Let's focus on the force and energy aspects. You've heard of the states of gas, liquid, and solid. The reason for these states is the force of attraction between the atoms or molecules. The stronger the attraction, the more likely it becomes a solid. Methanol becomes a vapor (a gas) very readily. Ethylene glycol stays a liquid even when hot. Mannitol is a solid. |
|
MELTING POINTS Methanol: -96 degrees Celsius Ethylene glycol: -12.9 degrees Celsius Glycerol (glycerin): 18 degrees Celsius Erythritol: 121 degrees Celsius Arabitol: 103 degrees Celsius Mannitol: 166 degrees Celsius |
5. Math: Here are the compounds from #2. Now we are using math to better understand these compounds. |
|
6. Building block: This molecule is called iron porphyrin. It's very similar to the molecule in hemoglobin. The "heme" in hemoglobin is referring to a molecule almost like this one. The green spheres here are carbon atoms. The blue ones are nitrogen atoms, and the small white ones are hydrogen atoms. In the center is iron (pink atom). The major building block for this molecule is a five atom ring (4 carbons[green] and 1 nitrogen[blue]). This 5 atom ring is called a "pyrrole". These are bridged to each other using one carbon atom with a hydrogen atom. One of these groups is circled. The molar mass of this one group of 5 carbon atoms, 3 hydrogen atoms, and 1 nitrogen atom is 77.0845 grams per mole. The molar mass the iron atom in the center is 55.8452 g/mol. 6. What the molar mass of the whole molecule?
|
|
|
For students in Phoenix College's CHM151 class, send your answers to me (Ken Costello) at chm151@chemistryland.com |
|
Visitors (not just hits) since Aug 2009