

| <-CHM151 Home Page | |
Quiz for Chemistry in a New Light |
|
| Copy each question below into an email and answer the questions there. (No need to copy the images). Mail your answers to your instructor at chm151@chemistryland.com | |
 |
1. You learned that chemistry involves 3 areas of focus: Building blocks, Force/Energy, and math. Let's say you are teaching your little girl how to make a cake. You will explain it to her using these 3 areas of focus. 1a: What building blocks will you mention that make a cake? 1b: What force or energy aspects will you mention? 1c: What math is involved in making a cake?
|
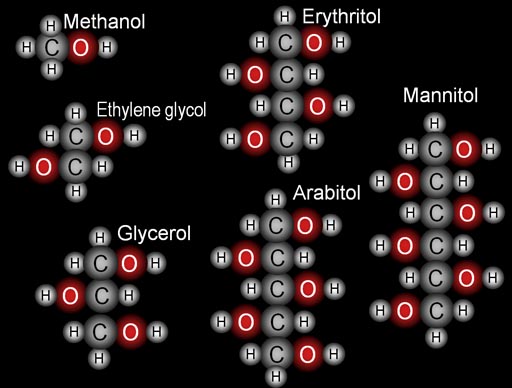 |
2. Building blocks: |
 |
3. Force and Energy: Here are three compounds from #2. Let's focus on the force and energy aspects. You've heard of the states of gas, liquid, and solid. The reason for these states is the force of attraction between the atoms or molecules. The stronger the attraction, the more likely it becomes a solid. Methanol becomes a vapor (a gas) very readily. Ethylene glycol stays a liquid even when hot. Mannitol is a solid. 3b. Which of these has the longest chain of carbons? |
|
MELTING POINTS Methanol: -96 degrees Celsius Ethylene glycol: -12.9 degrees Celsius Glycerol (glycerin): 18 degrees Celsius Erythritol: 121 degrees Celsius Arabitol: 103 degrees Celsius Mannitol: 166 degrees Celsius |
4. Math: Here are the compounds from #2. Now we are using math to better understand these compounds. |
|
5. Building block: This molecule is called iron porphyrin. It's very similar to the molecule in hemoglobin. The "heme" in hemoglobin is referring to a molecule almost like this one. The green spheres here are carbon atoms. The blue ones are nitrogen atoms, and the small white ones are hydrogen atoms. In the center is iron (pink atom). The major building block for this molecule is a five atom ring (4 carbons[green] and 1 nitrogen[blue]) bridged using one carbon atom with a hydrogen atom. Roll cursor over image to see this group. 5a. How many groups make up this molecule? 5b. Draw this molecule on paper (don't worry about colors). First copy what you see here, then try to draw it without looking. What tips would you give others about drawing this molecule from memory? |
|
.jpg) |
THERMITE: Some crazy neighbor shows you this reaction in his back yard. He calls it a "thermite" reaction. You want to do the same thing. 6. Keeping in mind "Chemistry in a New Light" and its 3 areas of focus, what questions might you ask so that you can repeat this demonstration and understand what is going on? Note: This picture is from www.amazingrust.com. Below is a link to a video of the thermite reaction from the website. It's in wmv format (a Windows video format):
For students in Phoenix College's CHM151 class, send your answers to me (Ken Costello) at chm151@chemistryland.com |
Visitors (not just hits) since Aug 2009