Below is the study guide for the mid-term exam. You have a 3 week window to come into the Testing Center and take the mid-term. Dates are from April 6 to April 27.
STATES OF MATTER |
| Classical states of matter are Gas, Liquid, Solid. Some non-classical are supercooled liquid (also called glass), liquid crystal, superfluid, and plasma. |
States of Matter |
Examples |
Properties of the State of Matter |
Shape |
Spacing |
Movement of Atoms/Molecules |
Compressible |
| Gas |
Air, helium in balloons, gasoline vapor, |
Expands to fill shape of its container |
Large |
Move at high speeds and random directions |
Yes |
| Liquid |
Liquid water, molten metals, solvents, solutions, liquified gases. |
Levels itself to fill its container |
Small |
Move at low speeds and random directions |
No |
| Solid (amorphous/crystalline) |
Metal, rocks, wood, sugar, bone, rubber |
Shape is fixed |
Small |
Vibrate but don't move |
No |
| Supercooled liquid (Glass) |
Glass windows, ceramic glaze, quickly cooled metals, sugar glass (used in stunts), obsidian |
Hard but shape slowly changes over many years |
Small |
Vibrate but barely move |
No |
| Liquid Crystal |
Molecules inside of liquid crystal displays (watches, computer screens, cell phones) |
Levels itself to fill its container |
Small |
Move in one direction but cannot rotate, so stay in semi-crystal formation. |
No |
| Superfluid |
A fluid that flows with no friction, so it can climb the walls of its container. Liquid helium. |
Levels itself to fill its container but then climbs out. |
Small |
Move at low speeds and but direction is not always random |
No |
| Supercritical fluid |
A liquid heated above a certain temperature and pressure acts like a gas and liquid. (supercritical CO2 decaffeinates coffee beans) |
Expands to fill shape of its container like gas but has solvent properties like liquids |
Medium |
Move at high speeds and random directions |
Yes |
| Plasma |
Ionized gas. Electrons and ions moving independent of each other in a gas state (The Sun) |
Expands to fill shape of its container |
Large |
Move at high speeds and random directions |
Yes |
On the exam, some of the cells above will be empty. You will fill those in. In the "Examples" column, you will only need to give one.
|
Four classifications of matter can be determined by asking 3 questions. Those four classifications are heterogeneous mixture, homogeneous mixture, element, or compound.
|
Matter |
|
|
No |
Is it uniform throughout? |
Yes |
|
It's a heterogeneous mixture (like sand and water) |
|
It's homogeneous matter. (well mixed) ↓ |
|
|
No |
Does it have a variable composition?
(more than one formula?) |
Yes |
|
Then it is a pure substance.
↓ |
|
Then it is a homogeneous mixture (like a solution or beverage, or any mixture of two or more substances that are thoroughly mixed) |
No |
Can this pure substance be separated into simpler substances? |
Yes |
|
Then it's an element |
|
Then it's a compound (one or more elements chemically combined) |
|
Using the questions in the above table, determine the classification of the following items: (Choices: heterogeneous mixture, homogeneous mixtures, element, or compound.)
Root beer float, sodium chloride (table salt), aluminum foil, grape juice, toothpaste, raisin bread, quartz, syrup, Pepsi on ice, filtered orange juice, orange juice with pulp, air, pure water, muddy water, ice made from pure water, plain paper, Christmas wrapping paper, 24 karat (carat) gold ring, 14 karat gold ring, iron nail, rusty iron nail, glass in a window, urine, NaHCO3 (baking soda). (These are answered at bottom of this study guide. First see if you can figure them out). |
More Classifications |
Write the numbers from the list on the right under the category that best fits the statement. Hint: There are three numbers (statements) for each word.
Atom |
Element |
Compound |
Molecule |
| |
|
|
|
|
1) A group of two or more atoms (same or different atoms)
2) A substance that cannot be divided into simpler substances.
3) A pure substance made from 2 or more different elements chemically bonded.
4) From a Greek word meaning indivisible.
5) The plural of the word means weather.
6) Consisting of two or more parts.
7) From two words meaning a small mass.
8) A structure made from protons, neutrons, and electrons.
9) Example would be NaCl
10) Example would be "copper"
11) Example would be a spherical object with nucleus and electrons.
12) Example would be the drawing on the left. |
Physical and Chemical Properties |
Physical properties are properties of the substance itself. Physical properties do not mention how the element or compound reacts with another substance. That belongs to chemical properties.
Some physical properties are color, form (gas/liquid/solid), odor, melting point, boiling point, malleable (easy to bend or reshape), crystalline, amorphous (no crystal structure), density, electrical conductivity (good conductor or insulator), index of refraction (how much it bends light), and more. Even without using equipment, you can identify the physical properties of substances such as color, form, taste, and odor. However, taste is not a good idea for checking most chemicals.
Physical properties of a natural diamond:
Color: Can be most any color.
Density: 3.5-3.53 g/cm3
Index of Refraction: 2.418
Hardness: 10 on the Mohs scale |
Chemical properties describe how a substance will react with other substances. For example, saying something is flammable is saying it will react with oxygen. So being flammable is a chemical property.
"Strong acids dissolve metals". This is a general statement of a chemical property for strong acids. Notice that it is mentioning a different substance (metals in this case). Chemical properties tell you how a substance will react with another substance.
"Sodium bicarbonate neutralizes acids." This is a chemical property statement for sodium bicarbonate.
"Citric acid is incompatible with alkaline carbonates and bicarbonate." A statement that uses the word "incompatible" is stating a chemical property. In other words, it is saying that this substance will react with these other substances. It's incompatible because citric acid will no longer stay citric acid if in contact with the other substances mentioned. |
| On the test, list 6 physical properties |
On the test, match the chemical property with the correct substance (Below are the correct matches. On the midterm exam, they will be scrambled. Some of the substances might be different, too.)
| Substance |
Chemical Property |
| Sulfur |
flammable |
| Potassium chlorate (KClO3) |
Oxidizer |
| Lye (NaOH/sodium hydroxide) |
Alkaline/neutralizes acids |
| Powdered iron |
easily rusts |
| Gold |
inert |
| Sugar |
digestible |
|
PHYSICAL AND CHEMICAL CHANGES |
| A physical change is when the physical properties of a substance have changed, but the make up (formula) of the substance does not change. For example, water changing to ice is a physical change. The physical properties of ice (such as density, hardness, index of refraction) are different than those of water. However, the formula for ice (H2O) is the same as for water (H2O). So there was no chemical change only a physical change.
Physical changes are usually easily reversible. |
A chemical change means the substance changes into another substance. In other words, its chemical formula changes. If a substance decomposes, that is a chemical change. If a substance reacts with another substance, that's a chemical change. For example, when the Statue of Liberty was erected in 1886, the copper metal was copper in color. However, by 1900, it had turned a turquoise color. The copper underwent a chemical change, going from metallic copper to a mixture of copper oxides, copper carbonates, copper sulfates, and copper chlorides due to exposure to various chemicals in the air and rain.
Chemical changes are not as easily reversible as physical changes. That's why we worry more about unplanned chemical changes. |
Physical Change Desired: Air Conditioning
The freon in air conditioners (home or car) changes from gas to a liquid and back to a gas, which then repeats. This is a physical change because the freon does not change its chemical makeup, just its state (liquid versus gas).
If freon leaks out, ultraviolet light in sunlight will decompose the freon. One decomposition product is chlorine gas. Is decomposition a chemical or physical change? |
Chemical Change Desired: Combustion for engines and stoves.
Whether it is combustion in the engine of a car or on the stove, combustion requires a chemical change. In these cases, it is a hydrocarbon (compound made from carbon and hydrogen atoms) that is combining with oxygen to form carbon dioxide and water. Energy is released and we get heat to build up pressure in the engine's cylinders or heat to cook our food. A chemical change is desired in this situation because that's what will produce heat.
If liquid gasoline enters the cylinder and simply turns to vapor but does not burn, is that a chemical or physical change? |
Physical Change Desired: Lighting
Most lighting depends on a physical change. The traditional light bulb has a filament that heats up and glows. That is a physical change. When the electricity is off, the filament cools down to its original physical state. However, if the bulb is cracked and air is introduced, the oxygen in the air reacts with the tungsten filament which then undergoes a chemical change as it becomes tungsten oxide, which is brittle. So the filament breaks and the light bulb stops working. In this situation, we don't want a chemical change. So the bulbs are engineered to prevent chemical changes.
Candles produce light. Is that because of a physical change, chemical change or both?
LED lights don't have glass enclosures to keep out oxygen. So the material that makes up LED is resistant to oxygen. Is that a physical property or chemical property? |
Chemical Change Not Desired: Food and Medicine Expiration dates
Why do food and medicine have expiration dates? It's because there is a slow chemical process going on. After the expiration date, a significant portion of the ingredients in medicine may have undergone chemical change and converted to some other chemical. Food has the same problem. Protein, fat, sugar, starches, and vitamins that make up food will chemically break down or react with other ingredients in the food or air. Bacteria will also alter the chemical makeup. So in these situations, spoilage or degradation is occurring because of chemical changes. Preservation of food and medicine is designed to slow down or prevent chemical change.
Chocolate is a food. If it melts, is that a physical change or chemical change?
Does cooking of food cause a physical change or chemical change?
A new bottle of Aspirin doesn't have much of a smell. However, over time it will smell like vinegar. Do you think a physical change or chemical change took place? |
METRIC SYSTEM |
| The Metric system is based on the Earth, water, and 10 fingers. These items we all have access to, so the metric measurements can be recreated by anyone on Earth.
The meter is the distance from the north pole to the equator divided by 10 million. This distance is a little longer than a yard. So we now have a distance measurement
A box that is one tenth of a meter (a decimeter) on all sides is called the liter. So we now have a volume measurement.
A liter of water is assigned the mass of one kilogram (1000 grams). So we now have a mass measurement.
The prefixes of the metric system are based on ten (because we have 10 fingers). Use the body to know these metric distances
One meter: Distance from neck to tip of fingers.
One meter: Distance between steps in a fast walk or when pacing out distances.
One decimeter=(0.1 meter): Width of the hand.
One centimeter=(0.01 meter): Width of a fingernail.
One millimeter=(0.001 meter): Thickness of a fingernail.
100 micrometers=0.1 millimeters=(0.0001 meter): The smallest object your eyes can see, which would be an object that is the width of a human hair. |
Use these average volumes related to the body to know metric volumes.
One liter: Volume of stomach when it feels full.
Half a liter: Volume of cupped hands.
Half a liter: Volume of air in normal breath.
5 liters: Volume of human head
5 liters: Volume of blood in body
5 milliliters (0.001 liters): Volume of eyeball
90 femtoliters (10-15 liters): Volume of one red blood cell
3 liters: Volume of water ingested per day
1.5 liters: Volume of urine output per day
|
Use these average masses related to the body to know metric masses.
8 grams: Mass of eyeball
65 grams: Mass of tongue
300 grams: Mass of heart
1.5 kilograms: Mass of brain
5 kilograms: Mass of head
100 kilograms (100,000 grams): 5% of American males are above this weight
87 kilograms (87,000 grams): Average weight of American male
3 kilograms: The mass one human hair can hold without breaking.
6,000 kilograms: If the hair on your head was woven into a rope, it could support 6,000 kilograms (the mass of about 3 automobiles.) |
| Regarding these questions about metric sizes that relate to the human body, the test will show the size and you will match it to the part of the body that is approximately that size. |
| The easiest way to do get rid of metric prefixes is to replace the prefix with the amount that prefix represents. For example, to change 54 milliliters to liters, change the "milli" to either 0.001 or 1/1000. So that means 54 x 0.001 or do 54 x 1/1000. Both get 0.054 liters. An easy way to add a metric prefix is to divide by what the prefix represents. For example, changing 5.5 liters to milliliters means divide by 0.001 to get 5,500 milliliters. |
Convert the following metric volumes: (On the test, the amounts will be somewhat different)
3000 milliliters to liters:
5 liters to milliliters:
half liter to milliliters:
1.5 liters to milliliters:
One trillion femtoliters to milliliters: |
Convert the following metric distances: (On the test, the amounts will be different)
2.35 meters into centimeters:
2.35 meters into millimeters:
1 centimeter into millimeters:
2 decimeters into meters:
0.35 meters into decimeter:
505 micrometers into meters:
|
Label-Factor Method (also called Dimensional Analysis) and Density problems.
This approach analyzes the dimensions (unit labels) of the problem and sets up a problem so that the starting dimension is changed to the answer by using a conversion fraction that cancels out the dimensions (units) we don't want and leaves (or introduces) the ones we want in the final answer.
|
10.0 mL x 19.3 g = 193 g
1 mL
50.0 g x 1 mL = 2.59 mL
19.3 g |
Here we see milliliters (mL) of gold being converted to grams (g) of gold by using the density of gold (19.3 grams per mL). The mL cancel out leaving just grams.
In the second calculation, we see grams of gold being converted to milliliters of gold by using the density of gold inverted. grams is on the bottom so that they will cancel the starting grams. |
| The below tables show the same calculations setup above. It simply shows it in a spreadsheet layout. The advantage of setting up problems in a spreadsheet is that the computer will recalculate the answer whenever you change any of the values. In other words, if the 50.0 g was changed to 144.6 g, the spreadsheet would display the new mL instantly. This of course depends on the formula placed in cell G1 having the formula "=A1*D1/D2". Notice the units in red get canceled, and the units in blue remain. Note: Sometimes column "C" with the "x" is left out, but it is still understood that multiplication of the fractions is taking place. Spreadsheets also let us use the row number and column letter to identify a particular cell. For example, A1 and B1 show the starting quantity. |
|
A |
B |
C |
D |
E |
F |
G |
H |
1 |
10.0 |
mL |
x |
19.3 |
g |
= |
193 |
grams |
2 |
|
|
|
1 |
mL |
|
|
|
|
A |
B |
C |
D |
E |
F |
G |
H |
1 |
50.0 |
g |
x |
1 |
mL |
= |
2.59 |
mL |
2 |
|
|
|
19.3 |
g |
|
|
|
The below table is the same as the above without the times "x" column.
|
A |
B |
C |
D |
E |
F |
G |
1 |
50.0 |
g |
1 |
mL |
= |
2.59 |
mL |
2 |
|
|
19.3 |
g |
|
|
|
|
| The below dimensional analysis setup (label-factor method) is similar to a problem in the tutorial. This setup converts 2 miles per hour into centimeters per second. Each conversion fraction have numerators and denominators that are equal to each other. These are written to cancel out the units we don't want (miles and hours) and get us to the units we do want (centimeters and seconds). Just like multiplying fractions, you multiply all of the numerators and divide by each of the denominators. You can skip any values of "1" because that doesn't change the quantity. So multiply 2 x 440 x 36 x 2.54 to get the numerator. Then divide by 0.25 followed by division by 60, then divide by the other 60. |
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
1 |
2 |
miles |
440 |
yards |
36 |
inches |
2.54 |
cm |
1 |
hr |
1 |
min |
= |
? |
cm |
2 |
|
hr |
0.25 |
mile |
1 |
yard |
1 |
inch |
60 |
min |
60 |
sec |
|
|
sec |
|
On the midterm exam, there will be some dimensional analysis problems that are set up that you solve. Some will be fully setup (like above) and some will be partially set up like below.
Driving 424 miles in a car that gets 22.0 miles per gallon will take how many liters of gas? The dimensional analysis is set up below to solve that, but you need to write the correct dimensions (measurement units) in their proper place. B1 shows that miles is the starting units. The information of 22.0 miles per gallon is inverted so that miles is in the denominator and cancels the miles in B1. At column E we have volume in gallons, but the question wants it in liters. So columns G and H convert the gallons to liters. So you would write "miles" in E2, "liters" in H1, and "gallons" in H2. Do the arithmetic to find the quantity of liters that is written in S1. On the exam, some units will be left out for you to insert into the proper cell. |
|
A |
B |
C |
D |
|
F |
G |
H |
I |
1 |
424 |
miles |
1 |
gallon |
3.785 |
liters |
= |
______ |
liters |
2 |
|
|
22.0 |
miles |
1 |
gallon |
|
|
|
|
| Ethanol is a common laboratory solvent and has a density of 0.789 g/mL. What is the mass, in grams, of 125 mL of ethanol? (Below is the partial setup to solve this problem. You will need to write the correct value and units in C1 and D1. Also, calculate F1. |
|
A |
B |
C |
D |
|
F |
G |
1 |
125 |
mL |
________ |
_______ |
= |
______ |
grams |
2 |
|
|
1 |
mL |
|
|
|
|
| Let's say you want to know the volume (in liters) of the iron in a car that gets crushed and melted down. The iron in the car weighs 3,155 lbs. Iron's density is 7.874 g/mL. The below dimensional analysis spreadsheet is setup. On the exam, some data will be left out. You should memorize how many lbs equal a kilogram. Do the arithmetic to find the quantity of liters (J2). Notice how D3 cancels B2. Notice E3 cancels the "k" in "kg" in D2. The strategy of dimensional analysis (label-factor method) is to let the units guide how you set up and solve these kind of problems. Notice that the density is inverted so that grams is on the bottom so that it cancels the "g" in "kg" in D2. "mL" is on the top because we want the answer in "Liters". On the midterm exam, you will have to decide where to place the measurements of density to get the answer. For example, the red text will not be there. |
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
1 |
Mass of iron |
convert lbs to kg |
cancel k |
Use density to convert grams to mL |
cancel milli |
|
|
|
2 |
3155 |
lbs |
1 |
kg |
1000 |
1 |
mL |
0.001 |
= |
______ |
liters |
3 |
|
|
2.205 |
lbs |
kilo |
7.874 |
grams |
milli |
|
|
|
|
| It's a sad fact but the United States is about the last country not to convert completely to metrics. This always adds some extra conversions in chemistry. For example, in a brewery the recipe calls for a concentration of malt of 2.5 grams malt per 100mL of brew in a vat that holds 1,500 gallons of brew. How many 25-lb bags of malt is needed? (equalities: 1 gallon=3785 milliliters, 1 lb=454g) Note the dimensional analysis below. It begins with the concentration, which is mass per volume. That it multiplied by the volume of the vat. Normally, that is all that is needed to find mass because the volume cancels. However, that only works if volumes have the same units and the mass is the unit you want. Unfortunately, our units for volume don't match (mL vs. gallons). Also grams is not the weight we want. We want the weight in the number of 25-pound bags. See below dimensional analysis that shows how to solve this. On the test, you will fill in columns G and H and solve for L2 (number of bags). |
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
1 |
concentration of malt |
Concentration x vol=mass |
Convert gal to mL |
convert g to lb |
|
|
|
|
|
2 |
2.5 |
g |
1500. |
gallon |
3785 |
mL |
1 |
lb |
1 |
bag (25lb) |
= |
______ |
25-lb bags |
3 |
100 |
mL |
|
|
1 |
gallon |
454 |
grams |
25 |
lbs |
|
|
|
The grams in B2 is canceled by what cell?
The mL in B3 is canceled by what cell? |
ENERGY |
 |
In the tutorial on energy, I said we can think of just two types of energy: Energy doing work, and energy waiting to do work. When it's doing work, we see it moving something, giving off some form of light, or changing the temperature of something like this train is doing. Work energy is also defined as a force applied to something which moves it across a distance. Like the force of lifting a brick from one height to a higher height. Or the force of a car pushing against the air as it moves down the highway. |
|
| Energy that is waiting to do work (has to the potential for work) is called potential energy and is recognized in 3 ways: |
| 1) It sits above ground level (like a lake behind a dam that sits higher than the land below the dam.) |
2) Something is compressed or stretched in someway (like compressed air or a compressed or stretched spring). |
3) Crowding of like charges or a separation of unlike charges (like a battery, capacitor, or electrons and protons on the same or different atoms. |
|

|
|
| Kinetic energy is the energy of something that moving. However, if that moving object is not pushing anything (like a spinning flywheel, a swinging hammer, or meteor flying through space), then this energy is just waiting to do work. So this energy is similar to potential energy yet it's not normally classified as potential energy. When these moving objects start to push on something, then they do work. Again, when that object moves freely, then the kinetic energy is waiting to do work. It's like potential energy in that sense that it has the potential to do work but hasn't done it yet. Again, when the moving object pushes on something and gets it to move. Then it becomes work energy, which is a force times the distance that force is applied. |
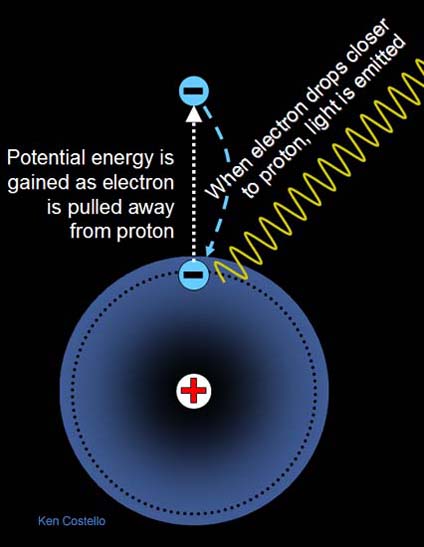 |
Chemical energy is a type of potential energy. It's similar to raising objects against gravity except instead of moving objects against the attraction of gravity, it is moving objects against the attraction of plus and minus charges. To illustrate this, let's look at the simplest of elements, hydrogen....
The hydrogen atom consists of one proton (+) and one electron (-). The proton sits in the center (nucleus) of the atom. The diagram shows the electron as a particle orbiting the nucleus. If an electron is moved farther away from the proton, it takes energy because they are attracted to each other (like gravity). The farther away the electron has been moved away from the proton, the more energy it takes, and the more potential energy stored in the electron. When the electron drops back closer to the proton, it will convert that potential energy to light energy. The light energy can be in the form of infrared, visible, ultraviolet, or even X-ray light.
Helium has two protons. Would you think it takes more or less energy to pull an electron away from the helium nucleus compared to a hydrogen nucleus?
Would an electron that has been pulled away from a helium nucleus have more or less potential energy than an electron that was pulled away from an hydrogen nucleus? |
| Check which items below possess kinetic energy |
Check which situations below are exhibiting energy doing work. |
[ ] Roller coaster stopped at the top of a hill.
[ ] A stretched rubber band
[ ] A candy bar
[ ] Water behind a dam
[ ] Wind
[ ] A car coasting down a hill
[ ] A car going up a hill |
[ ] Blowing up a balloon.
[ ] A car just sitting in the driveway.
[ ] A stick of dynamite.
[ ] Dynamite exploding.
[ ] Earth going around the sun.
[ ] Wind pushing on wind generators.
[ ] Talking
|
|
| Horsepower is work energy per minute. It came from the fact that one horse using a pulley could lift 330 lbs 100 feet in one minute. So that's 330lbs x 100 ft / 1 min. Or 33,000 ft·lbs/min. In other words, the pounds (lbs) times the feet divided by the minutes will come out to 33000 if one horsepower. We need an elevator to lift 1550 lbs. If this elevator gets 1550 lbs up to the 20th floor (200. feet) within half a minute, what horsepower motor is it using? Dimensional analysis uses the trick of letting the dimensions guide how to do the problem. Since horsepower has the units of ft·lbs/min, then it says to multiply the feet by the pounds, then divide by the minutes. On the exam, the values will be different and the problem won't be totally set up.
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
1 |
Distance lifted |
force to lift |
Divide by minutes |
convert to horsepower |
|
Answer in horsepower |
2 |
200. |
ft |
1550 |
lbs |
|
|
1 |
horsepower |
= |
______ |
horsepower |
3 |
|
|
|
|
0.50 |
min |
33000 |
ft·lbs/min |
|
|
|
ANSWERS:
Heterogeneous mixtures: Root beer float, , raisin bread, muddy water, Pepsi on ice, orange juice with pulp, Christmas wrapping paper, rusty iron nail
Homogeneous mixtures: Grape juice, toothpaste, syrup, filtered orange juice, plain paper, 14 karat gold ring, glass in a window, urine, air
Compounds: Sodium chloride (table salt), ice made from pure water, NaHCO3 (baking soda), quartz, pure water
Elements: Aluminum foil, 24 karat (carat) gold ring, iron nail |
| BALANCING CHEMICAL EQUATIONS |
You will be asked to balance the following equations: I will give the type of reaction just for additional information. At the end of these equations, I will go through the steps of balancing some of them.
(a) H2 + O2 --> H2O
Combustion reaction because of oxygen. It also qualifies as a combination
or synthesis reaction.
(b) C + Fe2O3 --> Fe + CO
This is a single replacement (displacement) reaction. The carbon atom(s)
replaces the iron. This is reaction shows how to turn iron ore into metallic
iron.
(c) H2SO4 + NaOH -->
H2O + Na2SO4
--> HOH + Na2SO4
This is a double replacement reaction and also called a neutralization
reaction. It's a bit hard to see the double-replacement. If water is written
as HOH, you can tell that the first H in water is the one that came from sulfuric acid and replaced the sodium (Na).
(d) Al2(CO3)3 --> Al2O3 + CO2
This is a decomposition reaction. Most carbonates (CO3) will
decompose to CO2.
(e) NH4I + Cl2 --> NH4Cl + I2
This is a single replacement reaction. The more reactive chlorine takes
iodine's place.
Balance the following equations: (I will comment on the reaction)
(f) MnO2 + CO --> Mn2O3 + CO2
Carbon monoxide is commonly used to strip off oxygen atoms from metals.
This above reaction shows the first step. If more CO is present, eventually
all oxygen atoms will be grabbed by CO and manganese (Mn) metal will be
left. This is how many metal ores get converted to metals.

(g) Mg3N2 + H2O
--> Mg(OH)2 + NH3
 The burning
of magnesium metal ribbons in chemistry labs is a common demonstration
(2Mg + O2 --> MgO). After burning, they point out the white
powder residue, and say that is magnesium oxide (MgO). Because air is
mostly nitrogen, there's also some magnesium nitride produced (Mg3N2).
If some water is added to the white powder, you will smell ammonia. That
can't happen if the powder is only magnesium oxide (MgO). You might stump
your lab instructor with that observation. The burning
of magnesium metal ribbons in chemistry labs is a common demonstration
(2Mg + O2 --> MgO). After burning, they point out the white
powder residue, and say that is magnesium oxide (MgO). Because air is
mostly nitrogen, there's also some magnesium nitride produced (Mg3N2).
If some water is added to the white powder, you will smell ammonia. That
can't happen if the powder is only magnesium oxide (MgO). You might stump
your lab instructor with that observation.
(h) C3H5(NO3)3 --> CO2+H2O+N2+O2

Whenever you see NO3 in a formula, watch out. It's often explosive
or at least creates oxygen to make something explosive. Also, whenever
a reaction produces a lot of gases like here, then it's usually an explosive.
Explosive power comes from a lot of gases being produced in a closed area
at a fast rate. The starting material here is nitroglycerin. Interestly,
nitroglycerin (diluted) is also used for medical purposes.
Extra Question: Go to this link and report on the medical use of nitroglycerin.
http://en.wikipedia.org/wiki/Nitroglycerin
(i) FeS + O2 --> Fe2O3 + SO2
Hint: When balancing this equation, balance the irons first, then the
sulfur. In equations with O2, alway save the O2 for last.
(j) Cu(NO3)2 --> CuO + NO2 + O2

Like mentioned on (c), nitrates are often a source of oxygen and belong
to a class of compounds called oxidizers. They are often used in fireworks.
Copper compounds are often blue and flames in contact with copper are
also blue.
(k) NO2 + H2O --> HNO3 + NO
Your automobile produces nitrogen dioxide because combustion in the cylinders
convert some nitrogen in the air to various nitrogen oxides. In contact
with water, nitrogen dioxide turns into nitric acid and nitrogen monoxide.
Both are unhealthy.
(l) Al + H2SO4 --> Al2(SO4)3 + H2
Many metals are dissolved by sulfuric acid. Acids have an "H+"
ion, which is short one electron. Many metals have their outer electrons
pulled off by the "H+" ion. When that happens, the metal dissolves.
This equation is somewhat simplified because it doesn't show these as
ions dissolved in water. Here is the full balanced equation with the ions.
You might use it to solve for the above equation:
2Al(s) + 6H+(aq) + 3SO42-(aq) -->
2Al3+(aq) + 3SO42-(aq) + 3H2(g)
(m) HCN + O2 --> N2 + CO2 + H2O
This equation is ironic. HCN is hydrogen cyanide, the poison used in the
gas chambers in World War II. If burned, the hydrogen cyanide is made
completely harmless.

(n) B5H9 + O2 --> B2O3 + H2O
This is where symbolic equations are very misleading. You can't appreciate the real chemistry. B5H9 is called pentaborane and is one of the most dangerous compounds around.
It's a powerful rocket fuel that the military nicknamed the Green Dragon
because of the green flame. Low doses can cause kidney failure and nervous
system damage. Exposure to the air can make it explode as well as any
hard bumps. Therefore, when balancing this equation, be extra careful
not to shake the table.

|
| How to balance some of the above chemical equations: |
(b) C + Fe2O3 --> Fe + CO
Here's how to balance this one. Look at
the "C" and see that there is one on the left side. On the
right side we see "CO" that also has just one carbon. So as far
as carbon atoms are concerned, the starting equation is balanced. We now
turn our attention to Fe2O3. Here we see two iron atoms (Fe2);
however on the right side, there's only one iron "Fe". To fix that we
write:
C + Fe2O3 --> 2Fe
+ CO
Right now, the carbon atoms and the iron (Fe)
atoms are balanced. We now look at the third element, oxygen. On the left
side there are 3 oxygen atoms, but the right side only has one inside of
"CO". To fix that we write "3CO".
C + Fe2O3 --> 2Fe
+ 3CO
This equation is balanced for Fe and for oxygen,
but by writing "3CO", we put the carbon atoms out of balance, so we
now write "3C" on the left side so that carbon atoms balance.
3C + Fe2O3 --> 2Fe + 3CO
This is the balanced equation because all
atoms started with are accounted for when the reaction is over with.
3C, 2Fe, 3O --> 2Fe, 3C,
3O They balance.
(c) H2SO4 + NaOH --> H2O
+ Na2SO4
--> HOH + Na2SO4
This is a double replacement reaction and also called a neutralization
reaction. It's a bit hard to see the double-replacement. If water is written as HOH, you can tell that the first H is the one that came from sulfuric acid and replaced
the sodium (Na).
H2SO4 + NaOH --> HOH + Na2SO4
Here's how to balance this
one. Start with the left compound and look at the hydrogen. H2 means 2 hydrogen atoms. This balancing is a little
tricky because these hydrogen atoms are what makes H2SO4 acidic. It's best not to mix these hydrogen atoms
up with the one on NaOH.
On the right side there's only one "H" atom. So we balance it by putting a two in front
of HOH
H2SO4 + NaOH --> 2HOH + Na2SO4
Right now, the acidic
hydrogen atoms balance. So let's go on
to the SO4 group (sulfate)
in H2SO4 The left side only has one SO4 and the right side only has one. So the SO4 group is balanced. We then move on to
"Na" in NaOH. There's only one sodium (Na) in NaOH, but on the
right side Na2SO4 has two Na atoms. To fix this we need 2NaOH.
H2SO4 + 2NaOH --> 2HOH + Na2SO4
What's left is the OH (hydroxide)
group. There's two on the left and two on the right, so those are
balanced. So here's the before and after count:
2H, 1
SO4, 2Na, 2OH --> 2H,
2OH, 2Na,
1 SO4 They all balance. So the above equation is the balanced equation.
(h)
C3H5(NO3)3 -> CO2 +
H2O + N2 + O2
You
start with the most complex compound, which is the one of the left. Let’s
start with balancing carbon atoms. We see there is 3 carbon atoms in C3H5(NO3)3,
so we need 3CO2 to have 3 carbon atoms on the right side.
C3H5(NO3)3 -> 3CO2 + H2O
+ N2 + O2
When we try to balance hydrogen atoms we see
that we have five on the left, but hydrogen in water on the right is in pairs (H2O).
So we have to double everything in order to balance 2x5 hydrogens on left and
5x2 hydrogens on right. That gives us 10 hydrogen atoms on both
sides. We will also have to double the carbon atoms on left and right
because we put a "2" out front of C3H5(NO3)3 in order to make 10 hydrogen atoms.
2C3H5(NO3)3 -> 6CO2 + 5H2O + N2 + O2
Now let’s balance the nitrogen atoms (Always balance oxygen last because it is
in more than one compound on the right). In (NO3)3,
there are 3 nitrogen atoms, but that is doubled because of the 2 in front of
nitroglycerin. So there are 6 nitrogen atoms on the left, which we can
balance with 3 N2 molecules on the right.
2C3H5(NO3)3 -> 6CO2 + 5H2O + 3N2 + O2
All that is left to balance are the oxygen atoms. The left side has 9
oxygen atoms in (NO3)3, but since there are two
nitroglycerin molecules, that is doubled to make 18 oxygen atoms currently on
the left side. On the right side we have 6CO2 + 5H2O
+ 3N2 + O2. The 6CO2 gives us 12 oxygen atoms, the
5H2O gives us 5 more, and then O2 is 2 more. That
adds up to 19 oxygen atoms. So we are not balanced. It would be
balanced if we just took ½ of O2 to get a single oxygen. Then
the right side would have 18 oxygen atoms. Sometimes an equation might
write ½O2 but that’s not normally wanted. So to get rid of ½O2,
we just double everything.
4C3H5(NO3)3 -> 12CO2 + 10H2O + 6N2 + O2
Now we have 36 oxygen atoms on the left and 36 on the right. The other
elements should still be balanced because they all got doubled equally.
(i) FeS + O2 --> Fe2O3 + SO2
Hint: When balancing this equation, balance the irons first, then the sulfur.
In equations with O2, alway save the O2 for last.
The number of oxygen atoms don't
match. When solving these save oxygen for last because on the right side
it is in two different compounds. Also start with the most complex
looking molecule,which would be Fe2O3.
FeS + O2 --> Fe2O3 + SO2
The Fe2O3 has 2 iron (Fe) atoms,
so we need to put a 2 in front of FeS on the left side so that the iron atoms
balance. We don't bother with the oxygen atoms just yet.
2FeS + O2 --> Fe2O3 + SO2
Now we look at the sulfur in "2FeS" and
realize we need 2 sulfur atoms on the right side, so we put a 2 in front of
SO2.
2FeS + O2 --> Fe2O3 + 2SO2
At this point iron and sulfur atoms balance, so we
have oxygen left to balance. On the right side there are 3 oxygen atoms
in Fe2O3, and there are 4 oxygen atoms in 2SO2. That's a total of 7
oxygen atoms on the right side. On the left side we just have O2.
We would have to have 3 and 1/2 O2 molecules to get 7 oxygen atoms. 3 1/2
O2 doesn't look that good, so we simply double it to get 7O2.
Because we doubled the oxygen atoms, we need to double all of the other
compounds. So we end up with. Now everything is balanced.
4FeS + 7O2 --> 2Fe2O3 + 4SO2
(j) Cu(NO3)2 --> CuO + NO2 + O2
The hint is to do the oxygens last. Another hint is to start with the
most complicated compound in the formula, which is Cu(NO3)2. Let's start
with copper (Cu). We have one on the left and one on the right, so as far
as copper is concerned, it is balanced. Now lets go to the "N"
inside of Cu(NO3)2. There are two nitrogen atoms because of the
parenthesis around the (NO3). So we have two N on the left side but only
one in the NO2 on the right. So we make it 2NO2, so that the right side
has two nitrogen atoms. So far we have this:
Cu(NO3)2 --> CuO + 2NO2 + O2
So far the Cu and the N balance. Now we tackle the oxygen at the end. The left
side has 6 oxygen atoms in (NO3)2. The right side has 7 (CuO=1,
2NO2=4, and O2=2). So now our oxygen atoms on the left side are too
few. The only way to increase them on the left side is to double the
Cu(NO3)2 to 2Cu(NO3)2. Well, now we have to start over again. We
have two copper atoms on the left so we have turn CuO into 2CuO, to get 2 Cu on
the right.
The same for "N". We now have 4 "N" on the left from
"2Cu(NO3)2". So we make the "2NO2" to
"4NO2" in order to have 4 "N" also on the right. Our
equation now is:
2Cu(NO3)2 --> 2CuO + 4NO2 + O2
Then we count the oxygen atoms again on the left.
"2Cu(NO3)2" has 12 oxygen atoms. On the right side our
"2CuO" gives us 2 oxygen atoms, the "4NO2" gives us 8. So
that totals 10. The O2 makes 12 oxygen atoms, so we are now done.
|
←CHM151Home page
|









 The burning
of magnesium metal ribbons in chemistry labs is a common demonstration
(2Mg + O2 --> MgO). After burning, they point out the white
powder residue, and say that is magnesium oxide (MgO). Because air is
mostly nitrogen, there's also some magnesium nitride produced (Mg3N2).
If some water is added to the white powder, you will smell ammonia. That
can't happen if the powder is only magnesium oxide (MgO). You might stump
your lab instructor with that observation.
The burning
of magnesium metal ribbons in chemistry labs is a common demonstration
(2Mg + O2 --> MgO). After burning, they point out the white
powder residue, and say that is magnesium oxide (MgO). Because air is
mostly nitrogen, there's also some magnesium nitride produced (Mg3N2).
If some water is added to the white powder, you will smell ammonia. That
can't happen if the powder is only magnesium oxide (MgO). You might stump
your lab instructor with that observation.


