Colligative (collective) Properties
Lowering concentration and
Creating Disorder

An ice storm is coming. Your car’s radiator has no antifreeze in it; all stores are closed, and you’ve got to use whatever you have around the house to keep the water from freezing in the car’s engine and splitting the engine block.
Rule one: What you add has to dissolve in water.
Rule two: If you add a solid or liquid that dissolves in water, it doesn’t matter what it is, just the amount.
Rule three: Just like antifreeze, your goal is to replace about ½ of the water with a solid or liquid that is miscible with water.
Rule four: It is generally best if the substance isn't volatile (doesn't evaporate easily).

Examples 1: Sugar, salt, baking soda, shampoo, laundry detergent, pancake syrup.
Examples 2: Rubbing alcohol, brake fluid
The items on the left are what you had. So you mixed all of these into a gallon of water and poured it into your radiator.
Note: This is where it doesn't matter what substance you use. It's the count (the moles) that matters, not its chemical makeup.
It was a long cold night and the next morning you wonder if what you did saved you thousands of dollars on a new engine.


Freezing point depression
Reason 1: The addition of these other substances reduces the number of water molecules that are available to freeze.
Reason 2: By adding these other substances, you’ve added disorder to the mixture. Nature tends to favor disorder (entropy). When water tries to freeze, it has to get organized, which will be more difficult because it’s mixed with all of these other molecules.

This frog is frozen but is still alive because the water in it did not turn into ice crystals, which would have ruptured the cells in its body. Why didn’t ice crystals form?
Glucose and glycerin in its blood and cells prevent water from freezing. In other words, glucose and glycerin get in the way of water trying to organize itself to create ice crystals. Also, their presence created disorder in the water. So water as to overcome that disorder in order to arrange itself neatly into ice crystals.

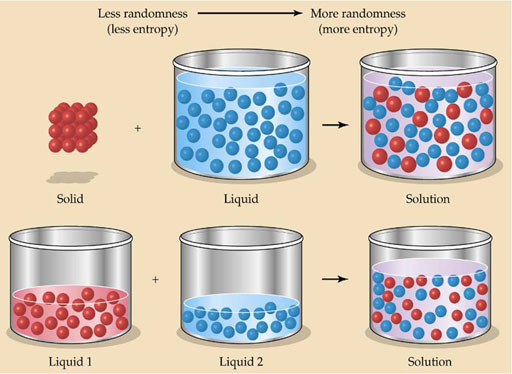
Pure substances are more orderly. When mixed, they lose that order and become more random (more entropy). Again, nature favors entropy.


Freezing Point Depression and Boiling Point Elevation
Antifreeze is also called a “coolant” because it can elevate the boiling point of water.
So, if you don’t have any antifreeze (coolant), what could you use instead?
Yes, the same items that you picked to keep it from freezing.

Let's talk more about freezing point depression.
Notice that ice cream melts differently than ice. Ice stays hard until it melts. Ice cream gradually get softer and softer.
Ice is a pure substance but ice cream is a mixture. In other words, the concentration of water in ice is 100%, but the concentration of water in the mixture is much less. So the 2 way movement of liquid water to frozen water favors frozen water because there are more molecules of water in the ice than out in the solution. So you have get colder than 0°C to get it to freeze.
About 30% of the water in ice cream never freezes because of the high level of dissolved solids like sugar, fats, and proteins.


Most anything will reduce its melting point if it is mixed with other substances. So be careful about adding eye drops to your eyes.

Pure molten silica (SiO2) freezes into quartz at around 2000°C, but if mixed with CaO and Na2CO3, it freezes at about 1000°C. Other additives can bring it down to 500°C. Actually, glass is classified as a supercooled liquid.

Eggs are mostly water, but dissolved proteins keep them from freezing at 0°C.
Chefs take advantage of this in frozen desserts.

OSMOSIS: Another Colligative Property

How do you make red blood cells go from what is shown in the left image to what is shown in the right image? On the right the blood cells have shrunk and have a spiked look to them.
Let's first look closely at the water on both sides of the cell wall (cell membrane) of the red blood cells.
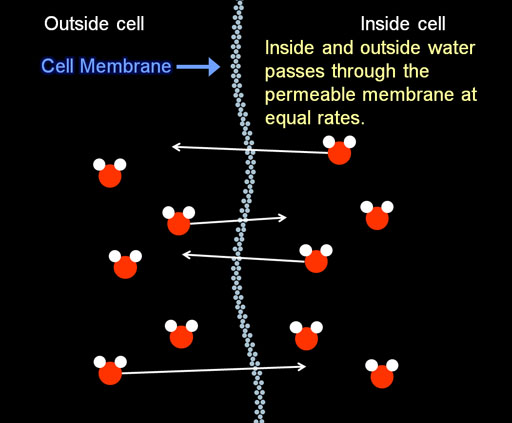
What we see is that water is passing through the membrane at equal rates. So that keeps the amount of water in the cell constant. So the cell does not swell up.
What happens if we add some salt (NaCl) to the solution outside of the red blood cells?
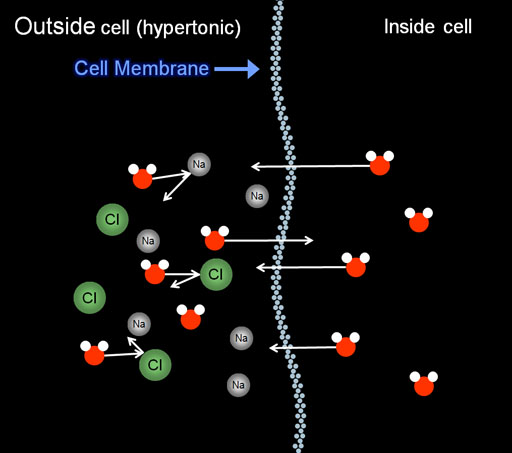
When the outside salt levels are higher than normal, it is called a hypertonic solution.
With extra salt ions gets in the way of water passing through the membrane. (You can see this is similar to how solutes (dissolved substances) prevent freezing. The solutes in both cases get in the way.)
As a result of being blocked by the salt and that there are fewer water molecules present, more water leaves the cell than gets into the cell. That causes the cell to shrink and look like the ones in the above picture. This is one example of osmosis. Osmosis is defined as a movement of a solvent or solute so as to equalize concentrations. Here pure water is moving to the high salt concentration making the salt less concentrated (more like pure water). If the salt could pass through the membrane, then the equalizing of concentrations would go even faster.

After measuring the change of height as 4.4 cm, you pipette 3 mL of the red solution out and weigh it. The mass is 3.75 grams. So the density is 3.75g/3mL or 1.25g/mL, which we can also write as 1.25g/cm3. Below I will calculate the osmotic pressure by finding the volume of the liquid and it's mass. Volume of that liquid is that of a cylinder. So V=πr2h. Height (h) is 4.4 cm. We didn't measure the tube's radius, but you will find we don't have to. Below is the sequence of the calculations to find the volume, then mass, then force, then pressure. (You don't have to know how to do this, but I wanted to show it because most books never show how the osmotic pressure is actually calculated.)


Another effect of having something dissolved in water is that it reduces the vapor pressure of water. That makes sense because the solute blocks water from trying to evaporate. This is similar to using antifreeze to keep water from boiling. One experiment that shows this was one I helped my cousin do for his 8th grade science project.
There are 2 plastic vials here. In one vial we placed distilled water. In the other vial we placed an equal volume of saturated solution of sugar. The caps on these vials were removed, but both vials were sealed in the larger jar. Water would evaporate from both vials and fill the jar with some water vapor. The water vapor would hit the surface of both the distilled water and the sugar water. The sugar, as you might expect, blocked water from leaving the surface of the sugar water. So over time, water that was in the vial with distilled water moved over to the vial that had sugar water.
Again, the explanation is that something dissolved in water will reduce water's ability to evaporate (reduce its vapor pressure). In this situation, the lowering of vapor pressure caused the sugar water to absorb the water vapor and, in essence, take the water away from the vial with pure water in it.
It's a pretty good trick.

I have a trailer near Show Low, Arizona which I have to winterize every winter. Instead of automotive antifreeze (ethylene glycol), I use propylene glycol because its not toxic. The trailer has a 30 gallon hot water heater. Temperatures in the winter get down to about zero degrees Fahrenheit (-18°C). How many gallons of propylene glycol do I need to add to reduce the water's freezing point to -18°C?
The textbook gives a simple looking formula for doing that.
ΔT = Kf msolute
ΔT is the drop in freezing point, which we want to be 18°C below its normal of 0°C.
Kf is a freezing point depression constant. It is different for different solvents. For water, the value is 1.86°C·kg/mol. What that means is the freezing point of water drops 1.86°C for each mole of solute (like antifreeze) that's in 1 kilogram of water.
msolute is the concentration measured in moles solute per kilogram of water. They call this molality, which is similar to molarity. Molarity is moles per liter of solution. Molality is moles per kilogram of water only (not solution).
So msolute is the moles of propylene glycol per kg of water. If we find moles of propylene glycol, we can turn that into grams, then grams into a volume. So let's solve for msolute:
ΔT = Kf msolute
Divide both sides Kf
ΔT = moles propylene glycol
Kf kg of water
If you multiply this by the kg of water in 30 gallons, you will get the moles you need.
ΔT = msolute
Kf
Put in the values:
18°C = moles propylene glycol
1.86°C·kg/mol kg of water
By multiplying by kg of water in water heater, we get moles of propylene glycol. See spreadsheet below for all calculations.
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
P |
Q |
|
| 1 | ΔT/Kf finds moles propylene glycol per kg of water |
Turns gallons of water into kg of water. After multiplying, we get moles of propylene glycol |
Use molar mass to get grams of propylene glycol |
Invert density to get mL |
convert mL into gallons |
gallons of propylene glycol needed | |||||||||||
| 2 | 18 |
°C |
30 |
gallons |
3.785 |
liter |
1 |
kg |
76.09 |
grams | 1 |
mL | 1 |
gallon | = | 21.3 |
gallons |
| 3 | 1.86 |
°C·kg/mol | 1 |
gallon | 1 |
liter |
1 |
mole | 1.036 |
g | 3785 |
mL | |||||
| 4 | |||||||||||||||||
21.3 |
gal antifreeze | 30 |
gal mixture | = |
12.5 |
gallons propylene glycol in 30 gal tank | |||||||||||
51.3 |
gal mixture | ||||||||||||||||
ΔT = Kf msolute
This formula is also very useful for finding the molar mass of an compound. Knowing molar mass helps you identify the compound. In the chapter 3 quiz, I had a problem where I said the molar mass of the sugar produced by a fungus was determined using freezing point depression experiment. Now, let's do that calculation. First let's solve the above formula for msolute.
msolute= ΔT
Kf
moles sugar = ΔT
kg of water Kf
Let's solve for moles of sugar
moles sugar = ΔT x kg of water
Kf
Let's say we added 22.50 grams of our unknown sugar to 55.74 grams of water and the freezing point of water dropped to -2.2°C. Remember for water the Kf is 1.86°C·kg/mol. Because Kf has kg we have to turn 55.74 grams of the water into kilograms. That's easy, that is 0.05574 kg. Plug these into the formula.
moles sugar = 2.2°C x 0.05574 kg water
1.86°C·kg/mol
°C and kg cancel leaving us with moles
moles sugar = 0.065929
How do we get molar mass? That's always grams per mole. So above we said we used 22.50 grams of the sugar. So we put that over the moles:
molar mass = 22.50 g
0.065929 mol
That reduces to 341.28 grams per mol. Since temperature was only measured to 2 sig figs, our best value for molar mass is 340 g/mol. When you look up the molar mass of the sugar maltose, these calculations show maltose as a good candidate for the unknown sugar.

Earlier I talked about how the sugar in regular coke reduced the freezing point of water in the can. The sugar used in the U.S. is normally fructose. The ingredients say 39 grams of sugar. Since water is the solvent, the Kf is 1.86°C·kg/mol. 12 oz is 355 mL. So the water present is close to 355 grams or 0.355 kg. The molar mass of fructose is 180.16 g/mol. Practice Problem 1: So how many degrees Celsius does the sugar reduce the freezing point of water in the can?
ΔT = Kf msolute

A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
P |
Q |
|
| 1 | ΔT/Kf finds moles antifreeze per kg of water |
Turns gallons of water into kg of water.
After multiplying, we get moles |
Use molar mass to get grams |
Invert density to get mL |
convert mL into gallons |
gallons of ethylene glycol needed | |||||||||||
| 2 | 6 |
°C |
3 |
gallons |
3.785 |
liter |
1 |
kg |
??? |
grams | 1 |
mL | 1 |
gallon | = | ??? |
gallons |
| 3 | 1.86 |
°C·kg/mol | 1 |
gallon | 1 |
liter |
1 |
mole | ??? |
g | 3785 |
mL | |||||

Hypertonic solution regarding blood is one that has more salt in it than normal blood. Isotonic is a solution that has the same salt concentrations as blood. Another name of isotonic salt solution is "normal saline"
Practice Problem 3: What is the concentration of salt in a normal saline solution?

Above and here I show a picture of red blood cells that were exposed to hypertonic solutions. They are all shriveled up.
Practice Problem 4: What do red blood cells look like that are exposed to hypotonic solutions where the solution has less salt concentration than what's in the blood cell?

Anything that can be is used to depress the freezing point of water can also be used to cause ice to melt. Here is your ice covered Porsche after an ice storm.
Practice Problem 5: What substances could you use to deice it?

In the Phoenix area, we are more worried about the car boiling over rather than freezing. So we want the water to boil at a higher temperature so that it is less likely to turn to steam and build up pressure. A similar formula to freezing point depression is used for boiling point elevation. The only difference is that Kb is the boiling point elevation constant. For water that constant is 0.51 C·kg/mol.
Practice Problem 6: If you have one gallon of ethylene glycol added to one gallon of water, what temperature will the water boil?
ΔT = Kb msolute
(Hint: Turn the one gallon of ethylene glycol into mL and then into grams (using density), then to moles using molar mass. You looked up those values in Problem 2. Turn the one gallon of water in kilograms.)

Practice Problem 7: Above, I talked about the wood frog hat could survive freezing. Read this article and report back what they learned about the wood frog which can be used to extend the shelf life of organ transplants.
http://news.nationalgeographic.com/news/2005/03/0301_050301_woodfrog.html