

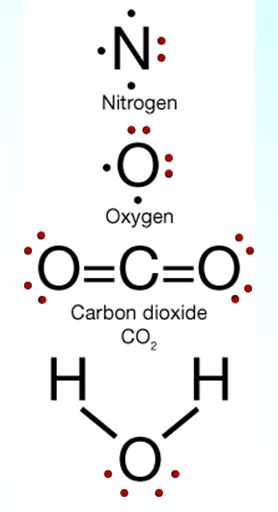
↑↓ |
In the Lewis dot structures on the left, I colored the electron pairs with red. The bonds are also electron pairs, but we are also interested in the ones not involved with bonding. They usually call these "lone pairs" or "non-bonding pairs" because they are not being shared with another atom. Notice the 2 lone pairs that's on the water molecule at the bottom. Even though they aren't involved in bonding, they do influence the angles of the bonds because they repel the electrons involved in bonding. So this is where the word "repulsion" comes from in the title (Valence Shell Electron Pair Repulsion). This is why the hydrogen atoms on the water molecule are not opposite of each other. The angle is bent.
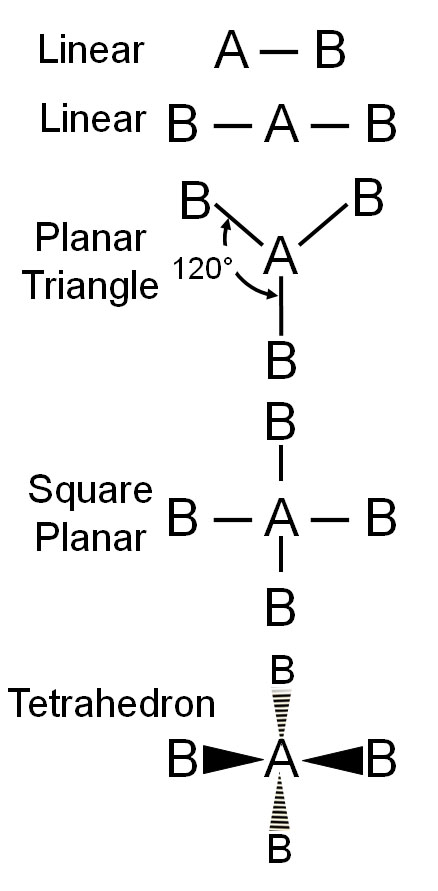
The whole theory of the lone pairs having repulsion capability came about because the shapes of molecules were not as expected. You know that electrons repel each other and get away as far was possible. Covalent bonds (2 electrons shared) will repel the other bonds. On the left is what we expect when atoms bond with each other. They try to spread themselves out as far as possible. Atom "A" is called the central atom. Atom "B" are the ones attached to the central atom. Since bonds repel, we expect these to be the shapes.
Linear: With B-A-B, we expect this to be a straight line (linear) because the bonds repel and 180° from each other is the farthest.
Planar Triangle: If you have 3 "B" atoms, they will spread out 120° from each other (3x120°=360°). This is called "planar" because "plane" means flat.
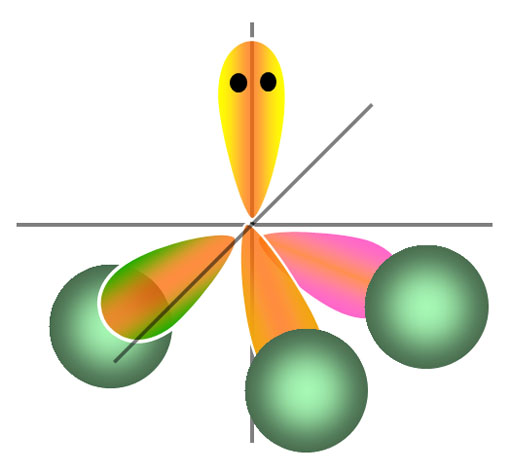
Square Planar: If you have 4 "B" atoms, then you might expect them to spread out like a cross. That's true in 2 dimensions, but in 3 dimensions, they can spread out even farther. They can do that by 2 of the "B" atoms going away from you (behind the screen) as shown by the striped triangles. The other 2 "B" atoms will get pushed forward towards you (in front of the screen) as shown by the solid triangles.
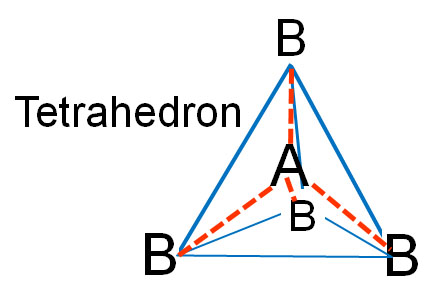
Tetrahedron: If you turn this last molecule around and set it on 3 of the "B" atoms, the "A" will sit above those 3 and the fourth "B" atom will be above the "A" atom. The bonds are shown in red. If you connect the 4 "B" atoms, you draw what is called a tetrahedron (blue lines), which means four-sided (four-faced) geometric shape.
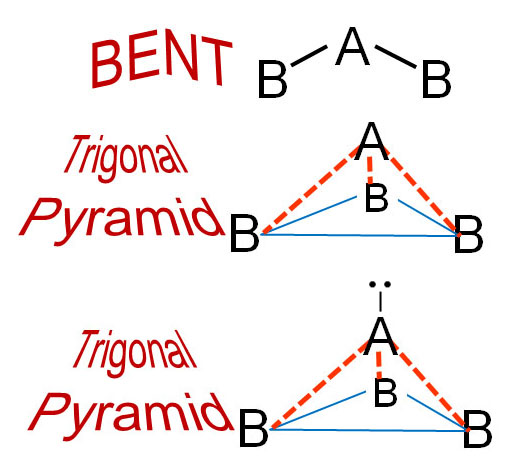
The above geometries are expected with molecules that have 1, 2, 3, or 4 atoms attached to a central atom. Some molecules do have these expected shapes, but some do not. For example some molecules with one central atom "A" and two "B" atoms, were bent. Something invisible was causing that. Chemists wanted to know why.
Another example was the occurrence where three "B" atoms didn't lay flat with 120° separation. They were pushed down away from "A" forming a pyramid with the "B" atoms forming a triangular base (This shape is called a trigonal pyramid). "Trigonal" is pronounced like "Trig" which rhymes with "trigger" (Trig uh nul).
The theory is a non-bonding pair of electrons on the "A" atom is causing a repulsion of the bonds between A and B pushing the B atoms downward.
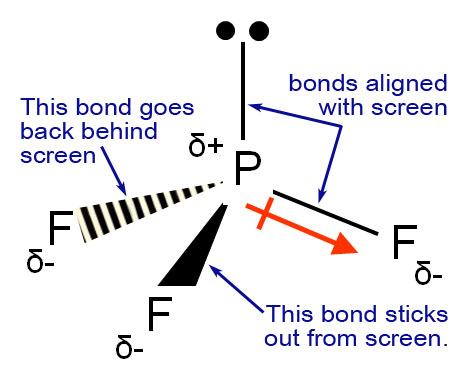
Brace yourself for some more symbols. At least you recognize the 2 dots that represent the loan pair of electrons. The "δ" symbol is the lower case of the Greek letter delta. The upper case delta is Δ. That usually means change. "δ" means "partial change in charge". "δ+" means a partial positive charge and δ- means, of course, a partial negative charge. This symbol ![]() means a separation of + and - charge. The left end that looks like a + sign is placed near the more positive end. The arrow → end is the negative end. In the picture the + end is the phosphorus atom. The - end is the fluorine atom. The reason for that is fluorine has a much greater pull on electrons than phosphorus, so the electrons are pulled towards the fluorine atoms. By the way, this degree of attraction to electrons is called electronegativity Each fluorine-phosphorus bond should show the
means a separation of + and - charge. The left end that looks like a + sign is placed near the more positive end. The arrow → end is the negative end. In the picture the + end is the phosphorus atom. The - end is the fluorine atom. The reason for that is fluorine has a much greater pull on electrons than phosphorus, so the electrons are pulled towards the fluorine atoms. By the way, this degree of attraction to electrons is called electronegativity Each fluorine-phosphorus bond should show the ![]() symbol. What this finally means is that this molecule is polar, meaning the phosphorus end is more positive and the end with the fluorine atoms are more negative. Being polar, tells us what kind of solvents this compound might dissolve in.
symbol. What this finally means is that this molecule is polar, meaning the phosphorus end is more positive and the end with the fluorine atoms are more negative. Being polar, tells us what kind of solvents this compound might dissolve in.
As mentioned earlier, the solid triangle means the bond sticks out towards you. The striped triangle means that bond goes back behind the screen. Those are symbols to help show you 3d with a 2d image. Note: sometimes the triangle is not solid, but means the same thing.
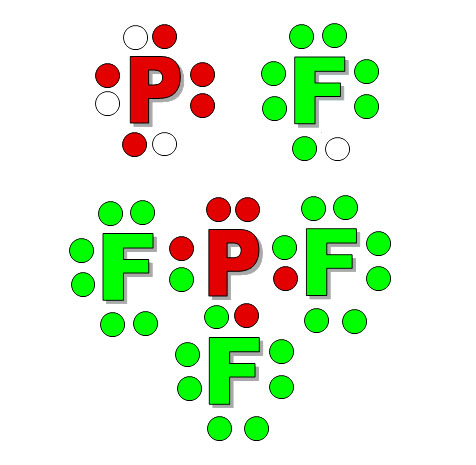
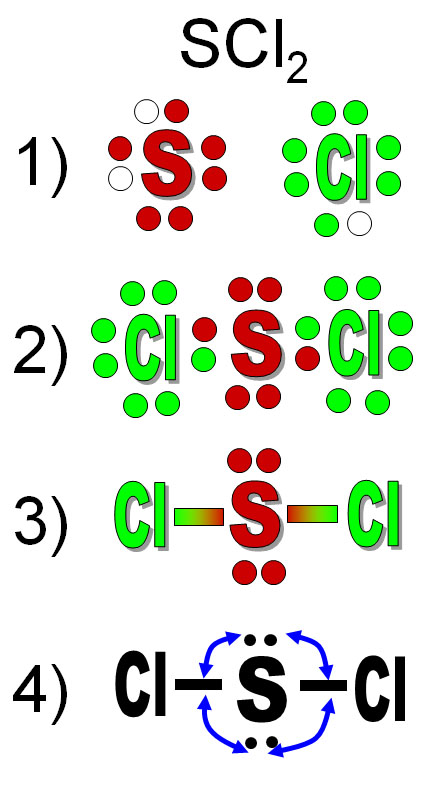
This is the process. They give you a formula and say, "What is the shape of SCl2 (sulfur dichloride)? "
1) Draw the elements with dots that match the number of outer electrons. Consult the Periodic Table and see what group (column) it belongs to, or go to Wikipedia and look up the element and find its electron configuration. Sulfur is in group 16 so subtract 10 for the 10 transitional metals and you get 6. Also, its electron configuration is given as ([Ne]3s23p4). The 2 & 4 show it has 6 outer electrons. That's why there are 6 dots around sulfur. It is 2 short of having a stable octet, so I put 2 empty dots. Chlorine is in group 17 (17-10=7), so it has 7 outer electrons. It is one short of having 8.
2) Combine the elements so that you get 8 around each.
3) The bonding electrons can be replaced with a line. Leave the lone pairs showing on the central atom. The non-bonding (lone pair) electrons on other atoms can be erased.
4) Think about the repulsion that the lone pair electrons will do on the bonds. If this was 2 dimensional, then the chlorine atoms would stay on opposite sides. However, in 3 dimensions, they get pushed towards the front and back.

5) The chlorine atoms will be pushed away from the screen and closer to you. The loan pair electrons will get pushed back behind the screen (The reverse is true also).
6) If you rotate the molecule so the chlorine atoms were flat with the screen, you could see that the bonds to the chlorine atoms are bent. So sulfur dichloride is a bent molecule.
As you can see this is a rather tedious process. If you make it to step 4, there are tables that tell you what shape the molecule will be. Below is such a table.
A |
B |
C |
D |
E |
F |
|
| 1 | Electron groups=bonds to the surrounding atoms + any lone pairs |
0 lone pair |
1 lone pair |
2 lone pairs |
3 lone pairs |
4 lone pairs |
| 2 | (2) The example shows 2 surrounding atoms and no lone pairs |
Linear |
||||
| 3 | (3) The examples show 3 surrounding atoms with no lone pair and then 2 surrounding atoms with one lone pair, which adds up to 3. The lone pair is like an invisible atom repelling the other bonds, so the molecule that is seen is bent. |
Trigonal Planar |
Bent |
|||
| 4 | (4) The examples show 4 surrounding atoms with no lone pair, 3 surrounding atoms with one lone pair, and 2 "X" atoms with 2 lone pairs. The shape of the molecule comes from looking at just the atoms, not the lone pairs. |
Tetrahedral |
Trigonal Pyramid |
Bent |
||
| 5 | (5) Here are combinations of "X" atoms and lone pairs that add up to 5. Look at the T-Shape. The "T" is turned sideways. Again, don't look at the lone pairs when naming the shape. The Trigonal Bipyramid uses the middle 3 "X" atoms as the 3-sided base. The top "X" is the peak of 1 pyramid. The bottom "X" is the peak of an inverted pyramid. |
Trigonal Bipyramid |
Sawhorse/SeeSaw |
T-Shape |
Linear |
|
| 6 | (6) Here are combinations of "X" atoms and lone pairs that add up to 6. The octahedral has 8 faces. It really is 2 opposing pyramids with a 4-sided base (square base). The Square Pyramid is similar but you don't consider the loan pair when you decide the shape. |
Octahedral |
Square Pyramid |
Square Planar |
T-shape |
Linear |
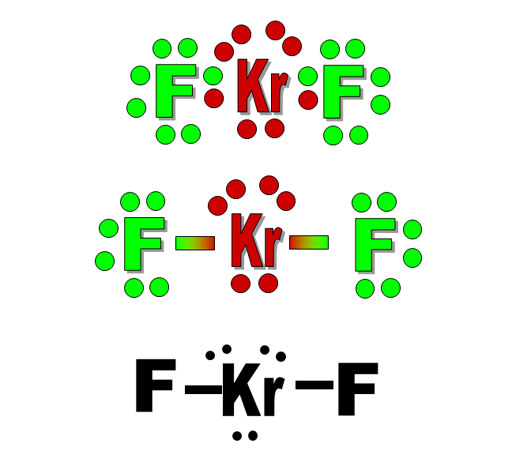
 <Top view
<Top viewHere is an example. We have the Lewis dot structure of KrF2 (krypton difluoride). Normally, atoms only want 8 electrons in their outer shell because of the limitation of 8 electrons in the s and p orbitals. However, the d orbitals sometimes gets used. So krypton can have 10 electrons in its outer shell (valence shell) by using d orbitals.
In the second image, the pair of bonding electrons are replaced with a line for the bond.
In the third image we can erase the non-bonding electrons around fluorines and just concentrate on the 2 bonds and the 3 lone pairs on the central Kr atom. That adds up to 5 electron groups. Using the table above, we find that cell E5 is the shape for 5 electron groups that have 3 lone pairs plus 2 surrounding atoms. The 3 lone pairs push on the fluorine bonds evenly, so the F-Kr-F molecule is straight (linear). The top view shows the 3 lone pairs forming a triangle. The Kr and F atoms are stacked in the center.
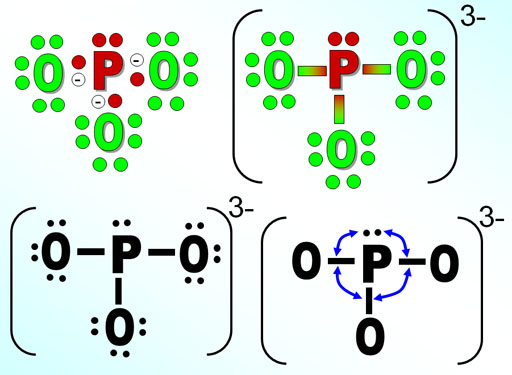
Practice Problem 1: To the left is the polyatomic phosphite ion. We want to know its shape. The upper left image shows the Lewis structure with the oxygen electrons (green), the phosphorus electrons (red) and the 3 extra electrons (white with - sign). In the upper right image, the bonding pairs of electrons are replaced with lines. The bracket shows the whole ion has a -3 charge.
The lower left image, simplifies it to black and white. The final image gets rid of the non-bonding electrons on the surrounding oxygen atoms. It lets us focus on the 3 bonds and the one lone pair of electrons on P (That's 4 electron groups). The blue arrows is to make us think of the repulsion between the bonds and the lone pair. Practice Problem 1: Using the table above, what shape is this molecule?

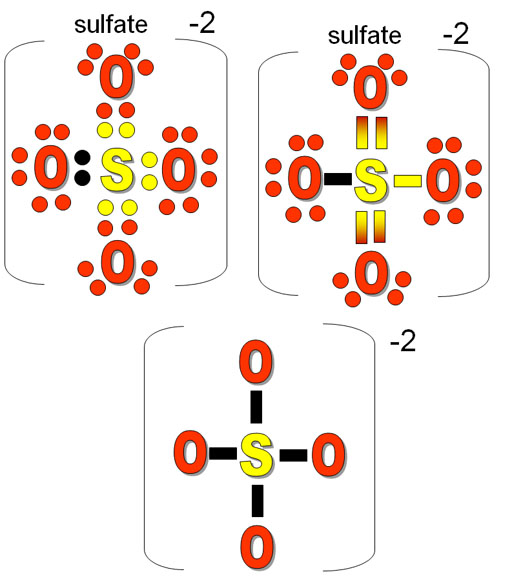
Practice Problem 3: The left image is the Lewis structure for the sulfate ion (SO4)2-. I like to use color dots to keep track of whose electrons are whose. Both sulfur and oxygen have 6 outer electrons. The black electrons are the extra 2 from somewhere else. That gives the ion the -2 charge. The right image turns the dots into bonds. I sometimes like to use a color gradient to still show where the electrons come from.
The bottom image is the simplified bonds that you use for considering the shape. Notice the double bonds are treated the same as single bonds. It shows the sulfate ion has 4 bonds to surrounding atoms (oxygen) and no lone pairs. So that's 4 electron groups. Practice Problem 3: Using the table, what is its shape?
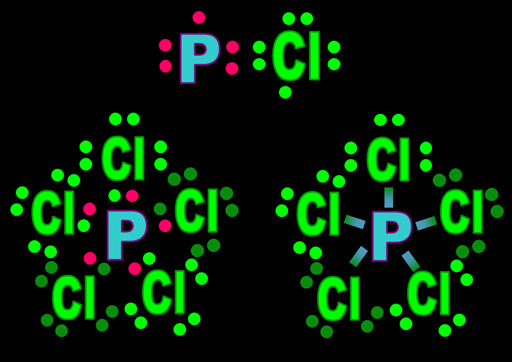
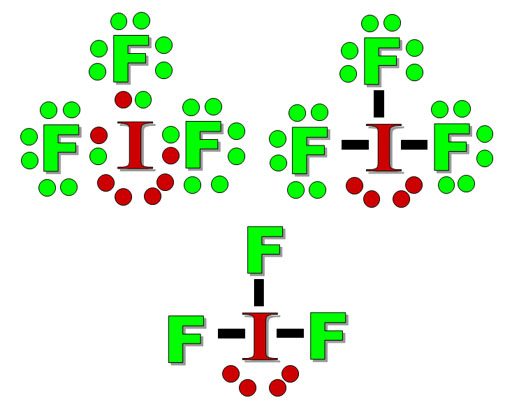
Practice Problem 5: The upper left image is the Lewis dot structure of IF3. Both fluorine and iodine have 7 valence electrons. You can see that the fluorine atoms have octets but the iodine atom has 10 electrons. The upper right image turned the pairs of bonding electrons into lines.
The bottom image has simplified the structure to show just the bonds and the 2 lone pairs on the central atom (iodine). Practice Problem 5: Using the table above, what is this molecule's shape?
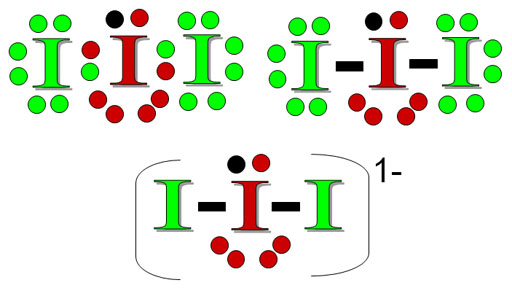
Practice Problem 6: This is I3-. The left image is the Lewis dot structure. This time the central atom is the same element as the surrounding atoms. Iodine has 7 valence electrons because it's in group 17 (17-10=7). The outer iodine atoms have an octet. The middle iodine has 7 of its own electrons, plus 2 shared by the other iodine atoms, plus the one extra electron from somewhere else. The right image turns the bonding pairs into bond lines. The bottom image simplified it for examining the number of bonds plus the number of lone pairs. Using the table above, what is the shape of I3-?