Doing chemical reactions is the main focus of chemistry because that's where the action is. That's when something gets made, changed, or destroyed. So a lot of emphasis is placed on chemical reactions.
Chemical reactions can be understood by these three areas of focus. A reaction is basically shuffling around the building blocks of chemistry (atoms/ions/molecules/electrons). Force & energy is what decides if the reaction occurs and how fast. Mathematics helps you keep an inventory of all the starting and ending materials.

As mentioned, chemical reactions mostly focuses on the building blocks. More specifically, how they are put together, how they disassemble, and ways to rearrange them.
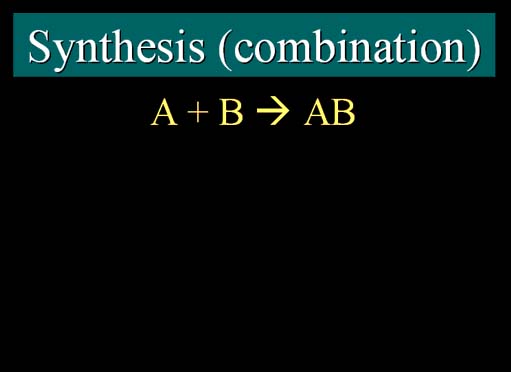
Synthesis (Combination)
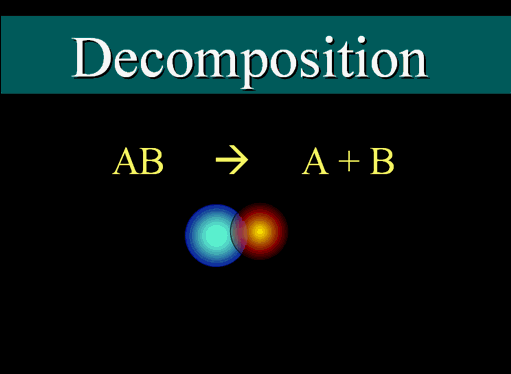
Decomposition
Single Replacement/Displacement
Double Replacement/Displacement
Oxidation (Combustion)
Synthesis (Combination)
Medicines / Drugs
Flavorings
Plastics
Man-made Jewels
Synthesis is the making of some compound such as a drug, a flavoring, a plastic, a jewel.
If atoms were big enough to pick up, we could easily make all kinds of interesting materials. Of course, they are extremely small and it's not that easy, but the concept is just as easy.
The synthesis reaction is normally represented by the letters "A" and "B" forming a compound represented by "AB". "A" and "B" could represent just two different elements, or they could be compounds (2 or more elements). (roll cursor over image for animation)
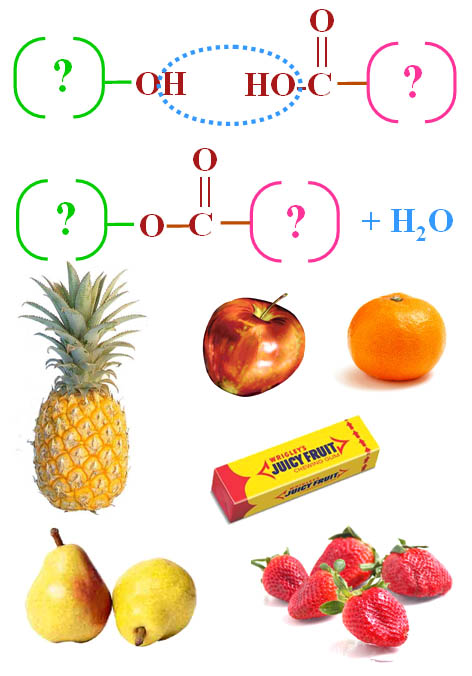
FLAVORINGS (example of synthesis):
Many artificial flavorings are made from combining an alcohol with an organic acid. An alcohol has an "OH" group. An organic acid has a carboxylic acid group (COOH). The questions marks means there is more attached to these groups. Depending what is attached, a different flavor is synthesized.
When the alcohol and organic acid combine, water is released. Below you will see some of the reactions that make the flavors you see here.
Notice that I show three ways of representing the molecules involved. The top are called "space-filled" models because they show the space that each atom takes up. You can see that the hydrogen atoms are smaller than the carbon or oxygen atoms. The middle models are structural formulas that help show the structure of the molecule. The bottom are skeletal structural formulas that indicate carbon atoms by a bend in a line. Carbon atoms are also at the end of line if there's no other element indicated.

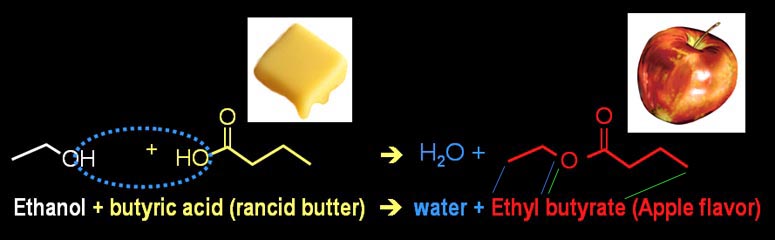
Below is the synthesis reaction for apple flavoring. Here we start with ethanol (a two carbon alcohol) and butyric acid (it gives rancid butter that bad odor). When combined they form ethyl butyrate. The name "ethyl" is used when there are a small chain of two carbon atoms attached. "Butyrate" is the butyric acid without its acidic hydrogen.
Below is the synthesis reaction for pineapple flavoring. When potatoes are fermented to make alcohol (ethanol), there's also some amyl alcohol produced (5 carbon alcohol). The smell or taste of amyl alcohol will make people instantly nauseated. The other reactant is salicylic acid, which is the active ingredient in many wart removers and anti-dandruff shampoos. Salicylic acid dissolves skin; however, when these two nasty chemicals are combined, they make a nice pineapple flavoring that you eat.

Decomposition Reactions:
Speed up decomposition
* Toxic waste
* Waste
* Fats
* Explosives
Slow down decomposition
*Anti-rusting
*Anti-aging
Decomposition Reactions:
We are interested in decomposition reactions for two different reasons. Sometimes we want to speed up decomposition and other times we want to slow down decomposition.

Here's an example of decomposition of turkey waste. We don't eat turkey feathers, heads, feet, and guts, so there's a need to get rid of this waste. Some companies have figured out ways to break down all of this waste into an oil. Remember most tissue (muscles, protein, etc.) in animals are made with very long chains of carbon. The process is to break down these long chains to about 10 to 20 carbon atoms long. That makes an oil similar to diesel or biodiesel.
The general formula for decomposition is shown here. This could mean two atoms that are bonded fly apart, or it can mean a larger molecule with multiple atoms coming apart. (roll cursor over image to see animation)
Potassium perchlorate is shown. When it decomposes it releases oxygen. That oxygen is used to speed up the combustion of material it's in contact with. For example, potassium perchlorate is used in fireworks. Other material such as paper or sawdust mixed with it will explode because of this source of oxygen.

Here's a fun decomposition reaction. The starting compound is ammonium dichromate. When heated, it begins to decompose into nitrogen gas, water vapor and powdered chromium (III) oxide. It looks like a volcano with ash being spread all over the place.
This looks like the compound is burning, but looking at the chemical equation we see no oxygen is being consumed. So the flame it produces does not need oxygen. So you can't put out this kind of "fire" by smothering it like most fires.
Here is ammonium dichromate again but this time showing a molecule of ammonium dichromate decomposing. (roll cursor over image to see animation).
Whenever you see gases in the products realize that the reaction can be dangerous because it can build up pressure and cause an explosion.
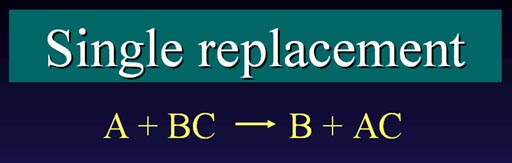
Single Replacement:
Another type of reaction is called single replacement
(or single displacement). One element that is not bonded to any other
element (A) comes in to take the place of another element that is bonded
to something (B). Element (B) is now freed.

Sacrificial metal:
An example of a single replacement reaction is when one metal is sacrificed
to save another metal. For example, concrete pillars have iron rebar in
them for strength. However, salt water can quickly react with the iron
to form iron (II) chloride. To prevent this, a metal like zinc or magnesium
is attached to the rebar and will protect the iron. Here's the reaction:
Zn + FeCl2 ->
Fe + ZnCl2
Notice the zinc metal becomes zinc chloride and the iron that had reacted with the chlorine becomes metallic iron. This preserves the iron rebar. In a similar manner the aluminum in boats are preserved by a piece of zinc or magnesium. See the person pointing to a plug of magnesium that is in embedded in the boat's rudder? That keeps the salt water from dissolving the aluminum; the magnesium dissolves in aluminum's place.


Neutralizing corrosive lye (sodium hydroxide).
Let's say you were using some drain opener (sodium hydroxide) and accidentally spilled some on the carpet. Lye is very dangerous and will cause blindness if gotten in the eye and will dissolve skin. The corrosive part of sodium hydroxide is the hydroxide. Notice that if you could replace the sodium in NaOH with hydrogen, you would get HOH, which is water. A weak acid like vinegar can supply that hydrogen. Below is the full reaction. Notice the water in the vinegar will dissolve the sodium hydroxide. The neutralization takes place when the H+ from the acetic acid bumps into the OH- from the sodium hydroxide. At that point they form water (HOH or written as H2O).


Oxidation reactions:
As the lists states, we are interested in oxidation reactions for two opposite reasons. Sometimes we want to speed it up and sometimes slow it down.

Hot air balloons use large propane torches to heat the air in the balloon. Unfortunately, that much flame can cause a catastrophic fire (see bottom image). Fortunately, the 11 people in this balloon survived.
All hydrocarbons (like propane) produce carbon dioxide and water as the combustion products. The unbalanced combustion reaction for propane is:
C3H8 + O2 --> CO2 + H2O
Since propane has 3 carbons, it makes 3 carbon dioxide molecules. Since it has 8 hydrogen atoms, it will make four H2O molecules. Let's show that:
C3H8 + O2 --> 3CO2 + 4H2O
Now we need to balance the oxygen on the left side. On the right side there are 6 oxygen atoms in the 3 carbon dioxide molecules and 4 oxygen atom in the 4 water molecules. That's a total of 10 oxygen atoms. Since oxygen travels as O2, we need five O2 molecules to give us 10 oxygen atoms. So our final balanced equation is:
C3H8 + 5O2 --> 3CO2 + 4H2O
AB -> A + B (decomposition)
A + BC -> B + AC
(single replacement)
AB + CD -> AC + BD
(double replacement)
Hydrocarbon + oxygen -> CO2 + H2O
(combustion/oxidation)