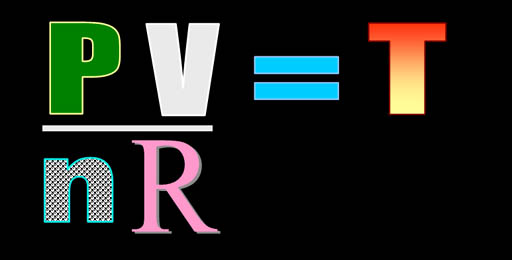|
PUTTING GASES
TO WORK |
|
|
These high tech
jet fighters take off from an aircraft carrier with a boost from one of
the oldest of technologies — steam. |
|
|
The secret to water and steam
power is that the pressure inside a closed container will get very high
when water is heated. That's because more and more water molecules will
go into a gas phase, which dramatically increases the pressure. Normally,
the pressure is used to push a piston like in the jet fighter launch above
or in a steam engine. If the pressure isn't released, the container is likely
to explode (roll cursor over image for animation). |
|
|
The very first engine was powered
by steam. It was invented by Hero of Alexandria, Egypt. Water was placed
in a sphere and then heated to boiling. Small tubes allowed the steam to
release. The openings caused an imbalance of the pressure in the sphere,
which spun the sphere. A similar engine is done with a thermos bottle filled
with liquid nitrogen. As the liquid nitrogen warms and turns to gas, the
escaping gas causes the thermos to spin. |
 |
A common misconception is that exhaust, whether it be
steam or flames, is the reason for the thrust and the propulsion. It looks
that way because that's where all the action is. However, how can flames
outside the missile actually do any pushing on the missile? Usually people
think that the flames are pushing on the air and that gives the missile
propulsion. But missiles work just as well in space where there's nothing
to push on.
|
|
|
To see what really gives the missile thrust,
let's look at a car that was designed to use steam propulsion. Whenever
there's pressure built up inside a chamber due to steam or exploding gases,
the pressure is pushing on in all directions and all
sides of the chamber. The chamber (or vehicle) doesn't move because
pressure is equal in all directions. But if
you have an opening on one side of the chamber, the fast moving gas molecules
causing the pressure have nothing to bounce off of (there's a hole there).
That means the the pressure on the side opposite
of the opening is not getting canceled
by any pressure at the exhaust opening. So the chamber gets pushed by gas
molecules hitting the chamber opposite of the opening (toward the front).
(roll cursor over steam car to see animation).
Realize it's the collisions of gases opposite
of the exhaust opening that pushes the vehicle
or missile. |
|
|
Using steam for propulsion like
jet propulsion isn't very efficient. The better way was have the steam pressure
push on a piston. Here are the basic components of the steam engine. First
there's the boiler, which is where you heat water and get a lot of pressure
built up. Next you open a valve to the piston cylinder, which let's in the
high pressure from the boiler. That pushes the piston up. At the top of
the stroke, you open a value to the condenser, which is cool and condenses
the water so it has no pressure. Outside air pressure will push the piston
down because there's very little pressure left in the piston's cylinder.
(Roll cursor over image to see animation) |
|
|
In the early days of steam engines,
they were such a novelty that people were charged admission to come look
at a steam engine powered train. |
 |
Steam engines were quite the work
horse in factories and on the farm. Anything that could burn could be used
to turn water into steam. Once you had steam pressure you could get it to
push a piston that would then turn a wheel. The large wheel (pulley) on
top was fitted with a large leather belt. The belt could be used to spin
another pulley that was attached to a saw, a pump, or whatever the factory
or farm needed to power. It was quite versatile. The drawback was all the
smoke from whatever fuel was used to heat the water. A new engine that had
much less smoke exhaust was the gasoline engine. |
 |
Gasoline Engine: The word "gas" is an alteration
of Latin, chaos. It's well chosen because when gas burns, the molecules
are in chaos.
Here's an article from 1897 talking about the new gasoline
engine.
"The gas engine is one of
the wonders of the 19th century. Now, within three years of the 20th century,
it is a novel machine, eagerly sought by many people. It is thought by
persons who have not studied its principles that it is a steam-engine,
using gas or gasoline as fuel for the purpose of making steam. This is
erroneous. Gas and gasoline in specific proportion with air are explosive
material."
"The expansive force derived
from explosion of these materials in the cylinder is the force that is
substituted for the expansive force of steam. Hence, owing to the economy
of this method as a means of deriving power, the steam engine and boiler
are fast disappearing, and the Gas Engine is taking their place for small
power." |
|
|
Even though the gasoline engine
created much less pollution than did the steam engine, there's still a lot
of pollution when there's so many cars. Here's Phoenix with its famous brown
cloud caused by nitrogen oxides coming from car exhausts particulary. The brown color is from NO2. |
|
Gases that Obey the Law |
|
A breakthrough about understanding how gases worked came in the 1700's. One such investigator was Robert Boyle (He must have had quite a hairdresser). He belonged to the "invisible College," which was a group of philosophers and science minded people who met on a regular basis to share their ideas and the results of their experiments. They were not part of any established university or college.
Boyle investigated many aspects of chemistry but by working with another member of the invisible college (Robert Hooke), they made improvements on a newly invented air pump and was able to do more experiments with air. One such experiment was about pressure and volume. Even though Boyle wasn't the first to recognize pressure and volume were related, his experiments helped prove the relationship and this relationship is named after Boyle, and is called Boyle's Law. |
|
|
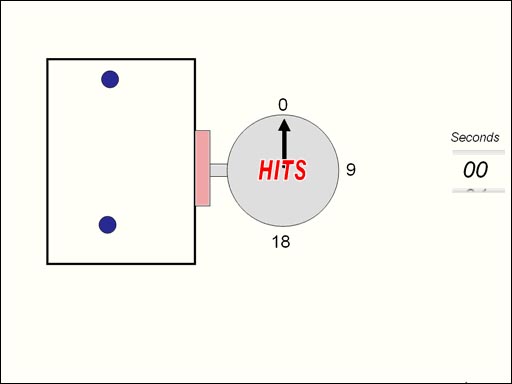
Before simply stating Boyle's Law lets take a microscopic look at air in a box. The molecules of gas are bouncing
around inside this box. There's a sensor that counts the hits. The hits
are proportional to the pressure. Next the box will get half its size and
the hits are counted again. What you see if that the hits are doubled if
the volume is halved. (Roll cursor over image for
animation). |
|
|
This law is actually quite logical.
If the volume decreases, the pressure increases
because as the volume (size) of the box gets smaller, the molecules of gas
don't have to travel as far to bounce into each other or the sides of the
box. So they bounce into each other and the sides more often causing increased
pressure. |
|
|
When one value goes up and another
goes down, they call that inversely proportional. In math they write it
as shown on the left. This says volume is inversely proportional to pressure.
If we multiply both sides by pressure (P), we get another equation that
also says pressure and volume are inversely proportional to each other. This is Boyle's Law of Gases. |
 |
Also, in the 1700's and early 1800's was the French scientist, Joseph Louis Gay-Lussac. He was a co-discoverer of the element, boron, and the discoverer of the element, iodine. He was also the one who developed the buret (burette) and the pipette used in chemistry. Now we see why these names are French words.
He also studied gases. |
 |
Gay-Lussac and fellow scientist Jean-Baptiste Biot did high altitude experiments using a hot air balloon. They wanted to know the moisture content at various altitudes along with the temperature. Their highest flight was 21,000 feet or 4 miles! That was in 1802, a hundred years before the first plane was invented, which only got about 10 feet off the ground.
It was his experimentation with temperature and pressure which brought about the law known as Gay-Lussac Law or the Pressure Law. |
|
|
This animation will reveal the concept. First the pressure is measured
at 81°F (27°C or 300K). We get 1 hit per second, which is an indication
of pressure. Now we heat up the gas in the box to double the temperature.
We count the hits again, and it has gone up to 2 hits per second, which
means the pressure doubled. (Roll cursor over image
for animation). |
 |
The above animation shows that pressure is directly proportional
to temperature. In other words, if temperature goes up inside a rigid container, pressure also
goes up. If temperature goes down, pressure must go down. Again, this is the Pressure Law or Gay-Lussac's Law.
A similar law is when gas and heated and the container the gas is in is allowed expand (like a plastic bag). It was shown that the volume is also proportional to the temperature. In other words, if temperature doubled, so would the volume. This is called the Charle's Law after Jean-Bapiste Charles who was give credit for it by Jospeh Louis Gay-Lussac. |
|
When this is combined with the knowledge that pressure is inversely proportional to volume, we get the bottom relationship. That says the product of pressure multiplied by volume is directly proportional to the temperature. |
|
|
In the previous tutorial I had the animation that
showed liquid water becoming water vapor with these extra water molecules
increasing the pressure. This demonstrates that pressure is directly proportional
to the number of gas molecules. This is called the Avogadro's Law after Amadeo Avogadro, another early investigator into the behavior of gases. (Roll cursor over
image for animation). |
|
|
Our formula is now factoring in
the influence of the number of gas atoms or molecules that is contained
in the volume. In many cases the gas is held in a container that can't stretch,
so when more molecules are added, the pressure has got to increase. (Roll
cursor over image for animation). |
|
|
In this animation, I will again
reinforce the idea that when volume goes down the pressure will increase,
and then the opposite, which is when the volume can get larger and larger,
the pressure will go very low. Again, this makes sense because the collisions
of the gases with themselves and the sides will get less and less as the
volume increases (roll cursor over image for animation) |
|
|
By using a conversion factor,
"R", we can set these values equal
to each other. This law is called the "Ideal
Gas Law" and explains how an ideal
gas behaves. This means a gas who atoms or molecules don't stick
to each other. They just bounce off like rubber balls.
In this formula, we will measure pressure (P)
in atmospheres (atm),
which is the pressure at sea level. Volume
(V) should be in liters
(L) . "n" represents the
count of the gas atoms or molecules in moles
(mol). "R"
is a constant that converts these units. To
do that it has a value of 0.0821 atm•L/mol•K.
"K" is the temperature
measured in degrees of Kelvin.
Simple algebra can be used to solve for each of these values. That way you
can solve for pressure, volume,
moles, or temperature
by knowing the other units. "R" is a constant so it doesn't really
need to be solved. |
|
|
Car talk is an entertaining radio
show that talks about car issues, but these car experts have a lot of chemistry
under their belt. One caller had a problem with the pistons that hold up
the hatchback to their car. To explain it, they mention the ideal gas law.
Click on link to hear this 3 minute dialog.
CarTalkAudioClip.mp3 |
|
|
Let's do a problem using the ideal
gas law equation. The question is we have a gas inside a one liter volume
and the pressure is 1 atmosphere. There's one mole of gas in the one liter,
so what must be the temperature? It must be cold because in earlier tutorials,
I said one mole of gas is 22.4 liters. However, I was assuming it was at
one atmosphere of pressure (14.7 psi=pounds
per sq. inch)
and 0°C. Here the pressure is the same, but it must be much colder for
the volume to shrink from 22.4 liters down to just one liter. |
|
|
To solve for temperature, we divide
both sides by nR. Then we plug in the values. |
|
|
Now we have 1 atmosphere for pressure,
1 liter for pressure, 1 mole for quantity, and the 0.0821 conversion constant.
Normally, I'd write all of the units, but for now let's keep it uncluttered.
So the math is pretty easy. Multiply 1 times 1 and divide that by 1 x 0.0821.
The answer turns out to be 12.2 K . That's close to absolute zero. That's
-261°C or -478°F. Like I said,
we expected it to be cold because the gas is in a volume over 22 times smaller
than normal. |
|
|
Let's do another problem that
has to do with dry ice evaporating inside a close container. As the dry
ice changes to vapor, pressure will increase dramatically inside of the
container. This technique is used to make dry ice bombs out of 2 liter soda
bottles. Fortunately, the bottle is plastic so the plastic shrapnel isn't
too dangerous, but the bottle cap can do harm. (Roll
cursor over image if you want to see an injury to the forehead when a bottle
cap blew off. Warning: it's a bit graphic).
That guy probably will think twice about doing that again. |
|
|
Here's the question: What
pressure could be reached when ¼ lb of dry ice is placed in this
2 liter bottle? Temperature is 86
°F (30 °C).
Since pressure is asked
for, the ideal gas law is solved for P. Now
we see that we need moles (n), temperature
(T), and volume. The problem says it's
a 2 liter container. It doesn't give moles,
but we can convert pounds to grams,
and then grams to moles. The temperature of 30°C
has to be convert to Kelvin by adding 273.
That gives us 303K. When we plug those 3 values
into our formula, we get the answer of 32.3 atmospheres. If we want the
answer is psi, just multiply by 14.7 psi/atm, which gives us 475 psi. That's
a lot of pressure. Most air compressor tanks only go up to 120 psi. A heavy
duty car tire will blow out at around 70 psi. So this bottle is definitely
going to blow up.
|
Here is the same problem written in spreadsheet format. We still want to solve the ideal gas law formula for P. So PV=nRT becomes P=nRT/V. The first cells are just to turn 1/4 lb CO2 into moles (n). The R constant has the units of atmosphere times liters divided by moles and divided by temperature in Kelvin. That's written as atm·L·mol-1·K-1 or atm·L/(mole·K).
| |
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
P |
Q |
| |
n |
R |
T |
/V |
|
= P |
| 1 |
pounds > grams |
grams > moles (n) |
R constant |
Temp in Kelvin |
Divide by volume |
Atm > psi |
pressure of CO2 |
| 2 |
1/4 |
lb |
454 |
g |
1 |
mole |
0.0821 |
atm·L |
303 |
K |
|
|
14.7 |
psi |
= |
475 |
psi |
| 3 |
|
|
1 |
lb |
44.0 |
g CO2 |
|
mole·K |
|
|
2.0 |
Liters |
1 |
atm |
|
|
|
Notice that the spreadsheet uses the formula as a guide for its layout. Be sure that if a term is divided then put it in the bottom row (denominator). Make sure all units cancel except the one that is in the answer. |
|
|
So far we mentioned pressure in atmospheres and in pounds
per square inch (psi). I'm sure you've heard of other units. The easiest
way to measure air pressure was to pour mercury into a tube that was about
a yard tall. Then put the thumb on the opening and turn it upside down
with the opening submerged in a bowl of mercury. You would think gravity
would just pull the mercury down; however, air pressure in the bowl pushes
on the surface of the mercury in the bowl which then pushes in all directions
including upward to hold the mercury up. At sea level the air pressure
holds the mercury 760 millimeters (29.9) inches above the surface of the
mercury in the bowl.
Instead of 760 mm, some people call it 760 torr
after a scientist named Evangelista Torricelli.
If water is used in the tube, air pressure can hold a
column of water that is 34 feet high no matter how large of a diameter.
|
|
|
By hooking a vacuum pump to a
container and pumping out the air (making a vacuum) you can get air pressure
to push the liquid up into the container. Remember a vacuum cannot "suck"
things into it. The lifting force only comes from the difference in air
pressure. The more air pumped out of the container, the bigger the difference.
Even if all air is pumped out, water will only rise 34 feet because at 34
feet water weighs 14.7 lbs. per sq. inch equaling the air pressure. I heard of a story of an engineer who forgot that fact and tried to pump water up a hill 100 fett using a vacuum pump. It didn't work. You have to use a pump submerged in the water and force it up the hill. |
|
|
For those of you going into a
medical field, you will undoubtedly see a device for measure blood pressure,
which is measured in millimeters of mercury. One of the more expensive types
actually uses mercury in a tube and the height of the mercury is how you
read the pressure. These devices are called either sphygmomanometers or
sphygometers. |
|
Gas Density |
 |
Gas Density is important to emergency responders because
they need to know if the toxic gas is going to be low to the ground, up
near the ceiling, or mixed with the air. Knowing the density tells them
that.
I've mentioned that a lot of these non-metal form compounds
that are toxic. It's smart to know if they are heavier or lighter than
air. The Periodic Table will help you calculate the density. |
 |
Air is mostly N2. So the grams
per mole is 14.01 x 2=28g. Since O2 is a little heavier, the density of
air is actually 28.8 grams per mole. Let's see if fluorine gas sinks or
rises in the air. Fluorine (F2) is 38.0 grams per mole (19.0 x 2). So it
will lay close to the ground, which means if there's a fluorine leak, run
out of the area on your tiptoes. Cl2 is 35.45x2=70.9g/mole, so it is very
heavy and will also be close to the ground. HF is (1.008+19=20.0). It will rise and be near the ceiling. So to
escape from an HF leak, crawl out of the room. All of these toxic gases can be done the
same way. |
| Congratulations on getting through this long
tutorial. |