
One of chemistry's best tricks is to determine the concentration of various substances. Imagine buying vinegar and sometimes the acetic acid concentration is so weak that it tastes like water, and other times it is so strong that it burns the skin off the tongue and throat. So finding concentration is an important skill.
Many substances that we use are either acidic or basic. Because acids and bases can neutralize each other, that gives us a strategy for finding the unknown concentration of an acid or a base when we know the concentration of the other one.


For example, in the last quiz, you did a problem about how lye (NaOH) and aspirin will neutralize each other. In other words, the OH- from NaOH will combine with H+ coming off of aspirin (acetylsalicylic acid) to make H2O.
Let's say we suspect the aspirin tablets do not contain 325mg of aspirin as they claim. If we trust that the NaOH is 100% lye as the lye bottle claims, we can use that to test the amount of aspirin in the tablets. One approach is to figure out equal quantities that will neutralize each other. We take 1 tablet of aspirin and see if the truly has 325mg. To know how many grams of NaOH it would take to neutralize this we have to count the aspirin molecules. So we use the molar mass of aspirin, which is 180.157 g/mole, to count the aspirin molecules. The count for NaOH needs to be the same if they are to neutralize each other. So we turn 325 milligrams of Aspirin into moles. That number of moles is the same for NaOH. Those moles get changed to grams of NaOH.

A |
B |
C |
D |
E |
F |
G |
H |
|
| 1 | ||||||||
| 2 | 325 |
mg Aspirin | 1 |
mole | 0.001 |
= | 0.00180 |
moles Aspirin |
| 3 | 180.157 |
g | milli |
|||||
| 4 | ||||||||
| 5 | 0.00180 |
moles NaOH | 40.0 |
g | = | 0.0720 |
g NaOH | |
| 6 | 1 |
mole |


Instead of using pH paper, we could add a pH indicator such as phenolphthalein. If the color of the solution is purplish, then there was less Aspirin than NaOH meaning there was less than the claimed 325 milligrams. If the solution is clear, then there is equal or more Aspirin than NaOH.
The problem with both of these approaches is they don't tell you how much less or more is the Aspirin compared to NaOH. So we need a better approach.
An improvement was to not to just dissolve the NaOH in water and dump it into a solution of the dissolved Aspirin all at once. The idea was to add it to the solution of dissolved Aspirin a little at a time. We call this method "titration". "Titrate" comes from "Titer/Titre" which comes from the same word as "title". A title can signify a certain quality like the title of "Premium, champion, first-class, etc". "titre" was a method used to report the quality of gold or silver. So the idea of determining the quality of gold or silver is similar to what we do now we use now to determine the quality (strength) of substance when doing titrations.
Above we calculated that 0.0720 grams of NaOH will neutralize 1 Aspirin tablet. Since 0.0720 g is too small to weigh out, we can make up What we could do is take twice that weight of NaOH (2x0.0720g), which is 0.144 grams and dissolve it in 50mL of water. We could then add this solution of NaOH to the solution of 10 dissolved Aspirin tablets. After adding 25mL, there will be 0.0720 grams of NaOH added, and all of the Aspirin should be neutralized. If it gets neutralized before 25mL, then that means there is less Aspirin than the 325mg expected. If it takes more than 25mL, then the Aspirin present is more than 325mg. The amount of mL more or less than 25mL would let us figure out the actual amount of Aspirin present.
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
|
| 1 | Converts g of NaOH to 0.00360 moles of NaOH | 0.0000720 moles per mL |
times mL added gives 0.001692 moles NaOH added |
1:1 neutralization |
0.001692 moles Aspirin converted to grams |
||||||||||
| 2 | 0.144 |
g NaOH | 1 |
mole NaOH | 23.5 |
mL | 1 |
mole Aspirin |
180.157 |
g Aspirin | = |
0.305 |
g Aspirin | ||
| 3 | 40.0 |
g NaOH | 50.0 |
mL | 1 |
mole NaOH |
1 |
mol Aspirin | |||||||
| 4 | |||||||||||||||

You then remember that when you left some of the NaOH granules on the table that they started to look wet. A few hours later, there was so much water that most of the granules were dissolved. So you realized they were correct.

So the problem is that the solution of 0.0000720 moles per mL (0.0720 moles per liter) of NaOH is only approximate. Is there a way to make up a solution of NaOH and then measure its concentration accurately? Yes, there is. It's by using a known amount of acid. One acid that is commonly used is called potassium hydrogen phthalate or KHP for short. This organic acid is a solid and easy to weigh out. It also doesn't absorb water so when exposed to the air, its weight doesn't change.
So the strategy is to first use the NaOH solution to neutralize a known amount of KHP. That will tell you just how much NaOH is in the solution. Then the NaOH solution with an accurately known concentration can be used to neutralize the Aspirin (acetylsalicylic acid) so we can get an accurate concentration of Aspirin.
To the left is the chemical equation for KHP releasing one hydrogen ion. That means each KHP molecule will neutralize one NaOH molecule because H+ + OH- --> HOH.


The procedure is to dissolve 0.3676 grams of KHP in about 50 mL of water and add a couple of drops of phenolphthalein to tell us when all of the KHP is neutralized. The solution will turn purplish as soon as there's just a quarter drop NaOH more than what is needed to neutralize the KHP. The KHP solution is put into an Erlenmeyer flask like show.
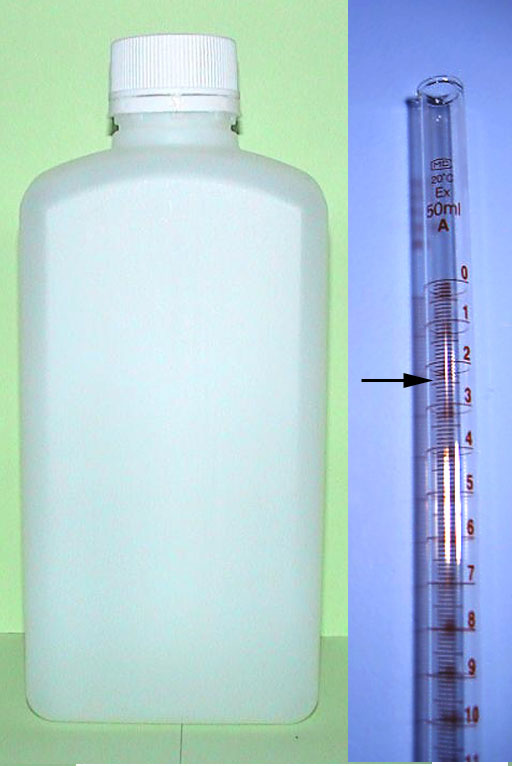
Previously we only made up 50mL of NaOH solution, but this time we want to have extra NaOH so we have enough to do titrations several times. So let's make a liter of NaOH solution. Our target concentration before was 0.0720 moles per liter, but let's just round that up to 0.1 mole per liter. It's weight then is 0.1 moles x 40.0 grams per mole, which comes out to be 4 grams of NaOH. We weigh out about 4 grams knowing that the weight isn't accurate because NaOH absorbs water. We then dissolve that in 1 liter of water giving us approximately 0.1 moles per liter and put it in a container that has a cap or stopper. CO2 will neutralize the NaOH if left exposed to long. We pour some of this into a 50mL buret and record where the starting volume level. Remember burets have zero at the top. Let's say the level of the NaOH solution reads at the 2.42mL mark (estimated just past the 2.4 line).

We then start adding the NaOH slowly to the flask with the KHP. It's basically let it flow a drop at a time. When it gets close to the point where all of the KHP is neutralized the clear solution of KHP will start to turn purplish but then the color goes away. Each drop added will cause the purplish color to persist longer. At one point the purplish color does not go away. That's when you stop and read the level on the buret. The level has dropped to 19.96 mL mark. The difference from 2.42mL and 19.96mL is 17.54mL. That's the volume of NaOH that has neutralized all of the KHP. Now you can calculate how strong your NaOH solution really is. We know it is in the 0.l mole per liter vicinity, but this pins it down more exactly.
Below are the calculations.
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
|
| 1 | Converts g of KHP to 0.001800 moles of KHP | Now we have 0.001800 moles of NaOH |
moles divided by liter makes moles per liter |
moles per liter | |||||||
| 2 | 0.3676 |
g KHP | 1 |
mole KHP | 1 |
mole NaOH |
= |
0.1026 |
moles | ||
| 3 | 204.22 |
g KHP | 1 |
mole KHP |
0.01754 |
L | L | ||||
| 4 | |||||||||||
Now we can return to out task of determining if these tablets truly have 325mg of Aspirin in them. We do the procedure just like with did with KHP, but this time our unknown is the Aspirin and our known is the NaOH concentration.
We place a tablet in about 50mL of water and add a couple of drops of phenolphthalein. We refill the buret with more our NaOH solution; however, this time we have an accurate concentration for the NaOH will will give us an accurate determination of the amount of acetylsalicylic acid (Aspirin) in the tablet. The top level of the NaOH solution is read to be at the 1.55mL mark (half way between 1.5 and 1.6 marks). We allow the NaOH solution to flow into the solution with the dissolved Aspirin at a drop by drop rate. As the purplish color appears we slow down the rate of the drops and then do a drop by drop addition of the NaOH solution. At the point we add a drop and the solution stays purplish (for 30 seconds), we then stop. The level is now at the 18.47 mL mark. That means 16.92 mL (18.47-1.55) of 0.1026 moles per liter NaOH solution was needed to neutralize the Aspirin.
See below for calculations
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
|
| 1 | Liters times moles per liter gives 0.0017339 moles NaOH | Now we have 0.0017339 moles of Aspirin |
moles times grams per mole makes grams |
moles per liter | |||||||
| 2 | 0.01692 |
L NaOH | 0.1026 |
mole NaOH | 1 |
mole Aspirin |
180.157 |
grams Aspirin | = |
0.3124 |
grams Aspirin |
| 3 | 1 |
L NaOH | 1 |
mole NaOH |
1 |
mole Aspirin | |||||
| 4 | |||||||||||

A |
B |
C |
D |
E |
F |
G |
H |
I | J | K | L |
M |
N |
|
| 1 | Liters times moles/liter gives 0.002792772 moles NaOH | Acetic acid loses 1 H+ ion. So we have equal moles of acetic acid (0.002792772) |
moles times grams/mole makes grams |
divide by mL of vinegar makes grams/mL | makes g/100mL | 5.59% w/v meanign 5.59g per 100mL | ||||||||
| 2 | 0.02722 |
L NaOH | 0.1026 |
mole NaOH | 1 |
mole acetic acid |
60.05 |
grams acetic acid | 100 |
= |
5.59 |
grams acetic acid | ||
| 3 | 1 |
L NaOH | 1 |
mole NaOH |
1 |
mole acetic acid | 3.00 |
mL | 100 |
100 |
mL | |||
| 4 | ||||||||||||||
So this titration showed that the vinegar bottle had a little more than 5% acidity. It had 5.59%.
Zumdaul: 6th Edition: pages 161-164, 7th Edition: pages 151-153
Tro: 2nd Edition: pages 158-159