plus The Art of Counting Without Counting

Before tackling the art of counting without counting, it's a good idea to go over the good news and bad news about chemistry.
It's like this guy. The good news is that he filled up with gas. The bad news is that the gas nozzle is still attached.

GOOD NEWS:
Chemistry has a simple motto about a simple truth: "It's
All About Building Blocks".
Actually, it's not all about building blocks, there's also force/energy, and math, but building blocks is always the best place to start.

Simple Combinations:
When atoms combine to make molecules, they combine in simple ratios, such as 1:1, 1:2, 2:2, 3:1, etc. There are no fractions.
Simple Connections:
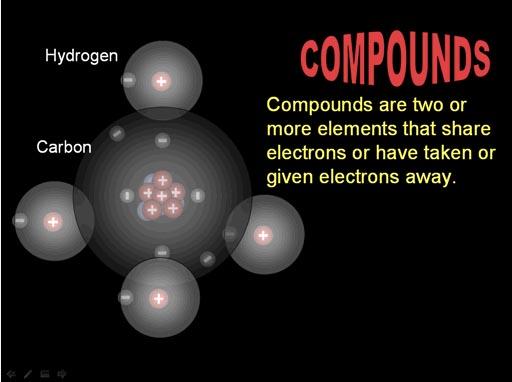
Atoms bond (connect) because they can either share electrons with another atom or the atom has taken or given away electrons.
Here the electrons of carbon and hydrogen are being shared.
(roll cursor of image to see animation)
Chemical Equations are simple:
Like we said, atoms combine in simple ratios. That means
the chemical equations that show and balance the starting "reactants"
and ending "products" are fairly simple.
Here we see how hydrogen and oxygen (reactants) react and produce water.
It's a simple rearrangement.
Roll mouse over image to see animation.

Good news. Your car did not fall into the water when it rolled off the dock. Bad news. A yacht stopped the fall but the damage to the yacht is more than the value of the car.
We've talked about the good news regarding chemistry, now the bad news.



Remember the counting of 100 grains of sand? That's not enough sand to do much. Even using 5 bags of sand to make a child's sand box would have too many grains of sand to count.

Let's consider a liter of water. That's not much water, but what if we had to count the molecules of water that was in that bottle of water? Since molecules of water are too small to see, let's say they were enlarged to the size of a grain of sand.
If the molecules of water in a liter of water were the size of grains of sand, it could cover the whole Earth 30 miles deep in sand. Imagine how many grains of sand that would have to be! That's how many water molecules you drink when finishing off a liter of water. It's a huge number.
(roll mouse over Earth to see it buried in sand)
So counting these water molecules is out of the question. There's got to be an easier way.
When counting a pile of coins, it would not be convenient to count them one by one. A smarter strategy is to weigh them. If you know the mass per penny, then you can count them without counting them. For example, a penny weighs 2.5 grams. If this stacked weighed 2,500 grams, then that means this stack contains 1,000 pennies.
In dimensional analysis we do it this way:
2,500 grams x 1 penny
= 1,000 pennies
2.5
grams
By the way, a nickel weighs 5.00 grams, which is easy to remember (5 cents / 5 grams).
To see a coin counter that counts by using the weight of the coins and by knowing the weight per coin, roll cursor over the pennies.


One of cargo containers contain a large number of skateboard
wheels. We are given this information. The total mass of the cargo container
is 5,000 pounds. The empty cargo container is 2,000 pounds. That means
the skateboard wheels must weigh 3,000 pounds. The weight alone doesn't
tell us how many wheels. We also must have the weight per wheel. They
tell us that 1,000 wheels weigh 100 lbs. That work's just as well. Here's
our calculation:
3,000 lbs x 1,000 wheels = 30,000
wheels
100
lbs.
Notice the "lbs" cancel and we are left with wheels.

Returning to our task of counting the water molecules in a liter of water, we know that a liter of water weighs 1,000 grams; unfortunately, we also need the weight per molecule of water in order to calculate the number of water molecules. For coins or skateboard wheels, we can pick up one and weigh it, but for a molecule of water, that's not going to happen. A partial breakthrough came a couple of hundred years ago.

Joseph Gay_lussac experimented with gases. He stated, "When gases react, the volumes consumed and produced are in ratios of small whole numbers (assuming they measured at the same temperature and pressure). Solids that react do not combine their volumes in simple ratios."
This was good news. Gases combine in simple ratios. Remember earlier we said atoms combine in simple ratios. There was something about gases that seem to count out the atoms in the gas so that they combined in even numbers.

Amedeo Avogadro had seen Gay_Lussac's lecture and was
interested in this unexpected fact about gases. For example, 2 liters
of hydrogen combined with one liter of oxygen to produce 2 liters of water
vapor.
To account
for these simple ratios (2 hydrogen per one oxygen) Avogadro theorized
that all elements in gas form must distribute themselves according to
number not weight. In other words, one liter of hydrogen has the same
number of molecules as a liter of chlorine or any other element in gas
form.
Even though we couldn't count these
hydrogen and oxygen molecules, we knew that we had twice as many hydrogen
molecules (2 liters vs. 1). This gives you its formula (H2O).
That's quite an accomplishment for something invisible.
In addition, by weighing equal volumes, he discovered that hydrogen weighed
1 gram and oxygen weighed 16 grams. That means the relative weight of
oxygen is 16 times that of hydrogen. That's another amazing trick for something that was too small to see or count.
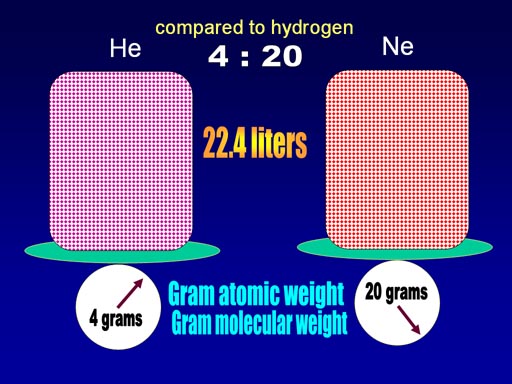
When the weight of helium and neon were compared to weight of a single hydrogen, helium was 4 times heavier and neon was 20 times heavier.
Avogadro found that if the volume of the gases were made 22.4 liters, then the weight of the gas happened to match the relative weight of the gas in grams. In other words, helium is 4 times heavier than hydrogen, and if we had 22.4 liters of helium, the weight is 4 grams. They called this the gram atomic weight (for single atoms) or gram molecular weight for molecules. Another name is molar mass which covers both.

Later the number of atoms or molecules in this 22.4 liters was given the name, "mole". The word "molecule" comes from "mole" and "cule". "Mole" means a mass, and "cule" means something small (like minuscule). So a molecule is a very small mass. The number "mole" gets its name from molecule.
Again, the "mole" represents the number of atoms or molecules in 22.4 liters of a gas. This number is also called "Avogadro's Number". Avogadro did not know this number. It was decades later that people started to calculate how many atoms or molecules there was in that volume. They honored Avogadro by naming the number after him.

Lord Kelvin (inventor of the Kelvin temperature scale) helped with calculating Avogadro's number. This number is also referred to as the mole.
It's a huge number, 6.022x1023.
602,200,000,000,000,000,000,000
This is the number of atoms or molecules of gas to make 22.4 liters (at 0 degrees Celsius and 1 atmosphere of pressure).

When you look at a Periodic Table, the numbers under the element symbols are the relative atomic weights. For example, notice helium and neon, it shows that neon is basically 5 times heavier (20.1797/4.00260). If we compare helium to carbon, we see that carbon is about 3 times heavier (12.0107/4.00260)
These are relative weights, but if we count out a mole of these atoms, then these numbers is the weight in grams . In other words, a mole of oxygen weighs 15.9994 grams (or round to 16 grams).

1,000 grams x 1 mole H2O = 55.51 moles
18.01528 grams

55.51 moles x 6.022 x 1023 molecules = 3.34x1025
1 mole
So now we've found a way to count the invisible.

The key to counting came from knowing the relative weights of the elements. Here is the list of elements used a couple of hundred years ago. Hydrogen was set as one. Oxygen was only listed as 7 times heavier because they didn't realize hydrogen gas traveled in pairs; otherwise it would be listed at 14 instead. Azote was the old name for nitrogen and it was thought to be 5 times heavier than hydrogen. Even these rough relative weights took over a thousand experiments of weighing, combining, decomposing, and reweighing.
The relative weights on the modern periodic table are quite accurate, but it took about 200 years and probably hundreds of thousands of careful experiments to arrive at this accuracy. But the payoff is that we can count the invisible atoms of any element and get the correct number to combine with other elements.


Again, the number below the element symbol is both the relative weight to other elements and also grams if you were to weigh a mole (6.022x1023) of atoms of the element.
A mole of helium weighs 4.00260 grams, a mole of boron weighs 10.811 grams, a mole of carbon weighs 12.0107 grams, and so forth.

Laughing gas, which is used as an anesthetic in dentistry, has the formula of dinitrogen monoxide (N2O). How many grams of nitrogen and grams of oxygen is needed to make one mole of N2O?
Well, a mole of N2O has one mole of oxygen atoms and two moles of nitrogen atoms. Since these are gases we've got two ways to measure out a mole of oxygen and two moles of nitrogen. First is by weight. The Periodic Table above shows that if we weigh out 15.9994 grams of oxygen we will have a mole of oxygen atoms. Earlier we learned that 22.4 liters of a gas is a mole of that gas. We could gather 22.4 liters of oxygen; however, since oxygen travels in pairs (O2), that would give us 2 moles of oxygen atoms, not one. So we simply cut the volume in half (11.2 liters). That's half a mole of O2 but one whole mole of oxygen atoms. The Periodic Table has 14.00674 as the relative weight or molar mass (mass of a mole) for nitrogen. Since we need two moles, we need 28.01348 grams or we can measure 22.4 liters. Nitrogen travels as N2, so a mole of N2 (22.4 liters) is actually 2 moles of nitrogen atoms.

C + O2 --> CO2
If there's not enough oxygen atoms, two carbons may share the O2 to make two carbon monoxide molecules.
2C + O2 --> 2CO
If you started with a pound of charcoal briquettes (carbon), how many grams of oxygen is needed to turn all of the carbon into the safe carbon dioxide?
 A
pound of carbon needs to be converted to a a number of carbon atoms.
A
pound of carbon needs to be converted to a a number of carbon atoms.454 grams x 1 mole = 37.8 moles
12.0107 grams


Since this is our equation, we see that we need twice the number of oxygen atoms. We counted the carbon atoms as being 37.8 moles. Oxygen will be twice that number, or 75.6 moles. The Peroidic Table says one mole of oxygen weighs 15.9994 grams. We need 75.6 moles.
75.6 moles O x 15.9994 g. = 1,209 g. Oxygen
1 mole
It's hard to picture 1,209 grams of oxygen (about 2.5 lbs). Remember 22.4 liters is one mole of O2 (2 moles of single oxygen atoms). We need 75.6 moles.
75.6 moles O x 22.4 Liter O2 = 847 Liters O2
2 moles O
That's 847 liters of pure O2. However, O2 in the air is only 20%, so we need five times as much air to have 847 liters of oxygen. So 5 x 847 liters = 4235 liters of air. That's 4.235 cubic meters of air (about the volume of an two-person tent. So if they brought the charcoal briquette fire into the tent to stay warm, the fire would have to use every bit of the oxygen in order to only produce carbon dioxide. If it did, there's no oxygen to breathe. If it left some oxygen and produced carbon monoxide, you'd be poisoned. So there's no good outcome here. The lesson, is don't bring a fire inside.

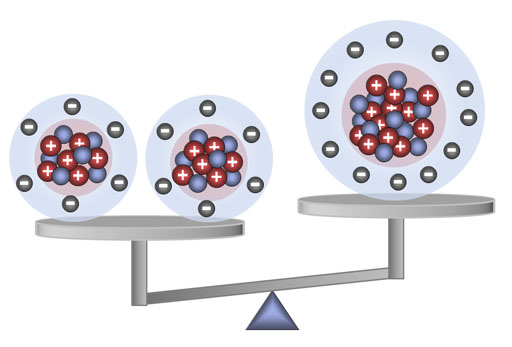
Carbon-12 has 6 protons, 6 neutrons, and 6 electrons. Magnesium-24 has 12 protons, 12 neutrons, and 12 electrons. So you would expect the mass of magnesium-24 would be exactly that of two carbon-12 atoms. Remember, all protons are alike, all neutrons are alike, and all electrons are alike. However, the mass of magnesium-24 is only 23.98504 atomic units. In other words, a mole of magnesium-24 is only 23.98504 grams. Where did the 0.015 grams go? The difference in the mass has to do with the difference in energy that it took to overcome the proton repulsion to make two carbon nuclei versus the making one magnesium nucleus. The average distance of protons in a carbon nucleus is shorter than the average distance in a magnesium nucleus. So it makes sense that it took more energy to push them closer until the strong nuclear force took over to hold them together. This extra energy is turned into mass according to Einstein's E=mc2 formula. So this is an example of the atomic world behaving different than what we are accustomed to.
STOICHIOMETRY
To summarize:
The good news. Chemical equations are simple with simple counting of the
elements or compounds.
The bad news. They are too small to count.
The new good news. We have a means to count them by weighing.
The art of counting elements is given the name "Stoichiometry". "Metry" means measurement and "Stoichi" is from Greek "stoikheion" meaning element.


The bridge between the mass and number requires the use
of the Peroidic Table to find out the mass of one mole of an element.
The bridge goes both ways. Starting with grams, you can count the moles
(or atoms) of the element. Or, starting with moles (a count), you can
find out how much that quantity weighs.
Motto: Use weight to count without counting and a count to weigh without weighing.
Behaviour change: When you hear "grams" you should automatically start to figure out moles. When you hear "moles" you should automatically realize you will probably be turning that into grams.