|
To evaluate Chemistry's impact, let's
use these 5 steps.
|
|
|
1. What did people want that chemistry could solve?
2. What was presented (unveiled) as the solution
to the problem?
3. What were the future consequences both positive and negative of the
solution?
4. What
was the history behind the solution both positive and negative?
5. What chemistry principles need to be learned to
better understand steps 1 through 4?
|
|
1.
What did people want that chemistry could solve?
|
|
|
Stone tool technology
allowed for the killing of large game and pottery
solved the problem of storing food in a weather and pest proof container;
however, the food itself could spoil because of microbes in the food.
People wanted ways of extending the storage life
of foods.
|
|
2. What was presented
(unveiled) as the solution to the problem?
|
| Various methods were discovered and presented
that would increase the storage life of food. Let's look
at the majority of them starting with the simplest. |
|
|
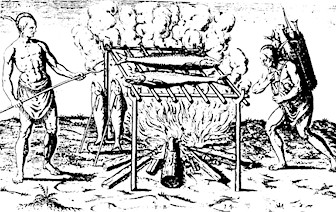
Cooking was one of the best ways to reduce spoilage.
Cooking kills bacteria and dries the food making the food less suitable
for bacteria to grow.
|
|
|
Additional drying with the sun and air would improve
the life of food. Here is beef jerky, which lasts quite a long time due
to its dryness and salt content. Exposing to smoke also dried and preserved
meat and fish.
|
 |
Meats and other foods packed in salt will
dry the food and kill microbes as well. For the same reason part of the
preservation of mummies was to spend a few weeks packed in salt. Even though
meat and fish packed in salt last a long time, when it comes time to eat
it, it is a good idea to soak the food to extract and discard the extra
salt. |
 |
When we think of buying fish, we expect them
to be frozen, chilled, or on ice. In China, the fish are dried and then
just hung up. |
|
|
When grapes are sun dried, they become
raisins, which last quite awhile. If not dried, they will develop mold.
|
 |
Milk as you know spoils quickly; however,
if it is separated into butter, cream, water, and milk solids (powdered
milk), it lasts longer. Powdered milk being dry lasts a long time. Even
butter if kept cool can have long storage. |
|
|
Milk can also be made to last by turning
into cheese. Certain bacteria and molds convert the milk to cheese and in
doing so create acids that kill the microbes that would spoil the milk or
cheese. Making wine uses a similar strategy. |
 |
Yeast is added to grape juice. When the yeast
digests the sugars in the grape juice, its byproduct is alcohol. Alcohol
kills other microbes that would spoil the grape juice. So the grape juice
is preserved in the form of wine. |
 |
If air gets to the wine when it is aging,
another bacteria called Acetobacter begins to grow and it will convert the
alcohol to acetic acid (vinegar). Vinegar means "sour wine". In
the upper right of the image, you see Mother of Vinegar, a slimy collection
of bacteria (including acetobacter) that converts wine to vinegar. Even
though this is bad for the wine, the vinegar formed can be used to preserve
other foods. |
 |
Vinegar
is used to "pickle" other foods. Cucumbers that are pickled are
called pickles, of course. But pigs feet, pig knuckles, eggs, and other
foods can also be pickled with vinegar. In vinegar these foods last a long
time because vinegar kills microbes. |
 |
Hot peppers are also known to kill microbes.
Food prepared with peppers have a longer shelf life. |
|
|
Even
in Middle-Age Europe, it was well-known that spices provide important
preservation qualities.
The most effective antimicrobial spices include garlic, onion, cinnamon,
cloves, thyme and sage. Cloves contain the oil, eugenol, also present in
sage and cinnamon.
|
 |
Allicin,
present in garlic, also acts as an antimicrobial agent, as does the allyl
isothiocyanate present in mustard. Thymol, present in thyme, oregano and
sage, is also noted for its antimicrobial properties.
|
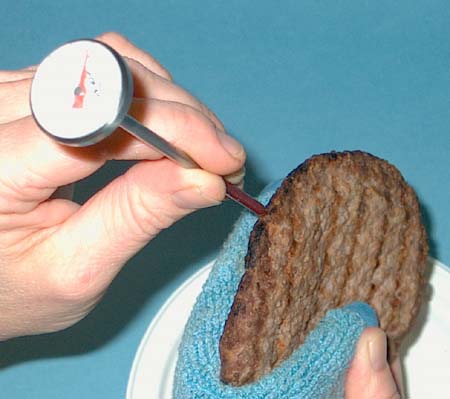 |
Research
at Kansas State University, Manhattan, has shown that cloves have a high
antimicrobial effect against E. coli in ground meat. Cinnamon, garlic,
oregano and sage were also shown to be effective.
60
people die each year from E. coli.
|
|
|
"More
than 600 people got sick in January 1993 from eating undercooked Jack
in the Box hamburgers contaminated with E. coli, a bacterium that can
cause diarrhea, abdominal pain and, in severe cases, kidney failure and
even death. Most of the victims were children living in Washington state;
four of them died."
|
 |
Most people think of soy beans as a health food. However,
it contains chemicals that are antinutritional, in other words interferes
with the proper absorption of vitamins and nutrients. Some researchers say
the ancient Chinese did not eat unprocessed soy beans. They treated the
soy beans in ways to extract or neutralize the antinutrients. |
 |
The antinutrients were handled in two ways. Fermentation
is a process where microorganisms breakdown sugars and starch in the food
to produce ethyl alcohol (drinking alcohol), lactic acid (found in yogurt
& cheese), and other compounds.
Soy sauce is from soybeans that undergo fermentation
from mold, yeast, and bacteria. The traditional process takes about 6
months and was developed over 4,000 years ago. These microorganisms add
additional proteins to the soy beans and destroys some of the antinutrients.
|
 |
Tofu from soybeans is another process that separates the
antinutrients from nutrients. It begins with soybeans being soaked, then
mashed, then made into a slurry. The slurry is filtered, which traps the
solid particles, but lets soymilk pass through. The soymilk is treated with
either lemon juice or a salt like magnesium chloride or calcium sulfate.
This causes the soymilk to curdle. The curds are what is pressed into tofu
blocks. The liquid portion (whey) is not kept for consumption (the antinutrients
are here). This process of increasing the nutrient value of soybeans is
about 2,000 years old. |
 |
Olives are another food that are made edible through chemistry.
If eaten from the tree, they are incredibly bitter. To make them edible,
they are soaked in water, salt water (called brine), or water that is made
alkaline. The soaking is for weeks or months. |
 |
Using just a little chemistry to extract the chemical
that makes olive bitter, we can enjoy a variety of olives and olive preparations. |
 |
Instead of making the olive edible by extracting the bitterness,
extracting the olive oil may be the goal. Extra virgin olive oil means the
extraction of oil is done by squeezing and without the use of heat or chemicals. |
 |
Historically, olive oil was extracted from olives by crushing
them first. This big stone had been used for centuries and is still being
used for crushing the olives. |
 |
The crushed olives were placed in round woven sacks that
were then squeezed with a large press. The olive oil would squeeze out of
the sacks and run into the trough below. |
 |
On a smaller scale, this carved rock was used to crush
olives and collect the olive oil. Olive oil provided much needed calories
for the ancient Greek people. It has also been shown to have many other
health benefits. |
 |
In modern factories, the approach is similar. Large stone
wheels crush the olives, and the olive oil is drained off. |
|
|
In summary, ancient people through trial and error found
ways to make food last longer and make plants or animals that were not edible,
edible. They didn't understand the full chemistry behind their success,
but they were learning and applying chemistry. |
| 5. What chemistry principles
need to be learned to better understand steps 1 through 4? |
|
|
The basic principle for preserving foods is to create
an environment that is not favorable to microbes.
Since most living things need water to survive, the
removal of water from food is a good way to prevent spoilage. Dehydration
is the process of removing water from foods. Cooking is a fast way to
remove water plus it kills bacteria that might be in the food.
|
|

|
When all water is removed, like these freeze-dried
foods of ice cream, pizza, lasagne, tamales, etc., the food will last a
long time without refrigeration. The containers are also airtight to keep
out moisture and bacteria. These are not dried with heat, but with a vacuum.
Even ice evaporates some, and in a vacuum it evaporates much faster. |
|
|
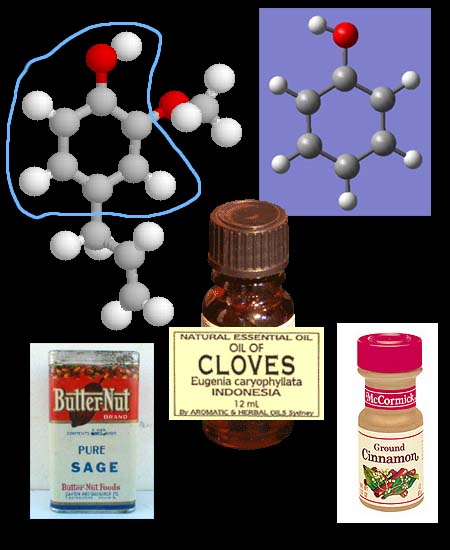
Spices
contain essential oils that have antimicrobial
properties. Many of these oils are derived from a compound called phenol.
In the upper right of the picture, the 6 gray balls are carbon atoms. The
small white balls are hydrogen and the red ball is oxygen. This molecule
is called phenol and is used as an antiseptic
and disinfectant. In plants, additional compounds
are bonded to the phenol to produce a wide variety of chemicals many of
which are antimicrobial. The other molecule
shown is eugenol, which is an essential oil
found in cloves, sage, and cinnamon. It has
good antimicrobial properties. The circled part of the molecule shows that
phenol is part of its makeup. |
|
|
Most microbes have difficulty living in water
with alcohol present. Yeast, which produces it, is more tolerant. How alcohol
kills bacteria and viruses isn't confirmed, but it is believed that the
alcohol alters the shapes of proteins, enzymes, and DNA in the bacteria
and perhaps dissolves some of the membranes. Because of the alcohol, wine
is much saver to drink than water in most parts of the world. |
|
|
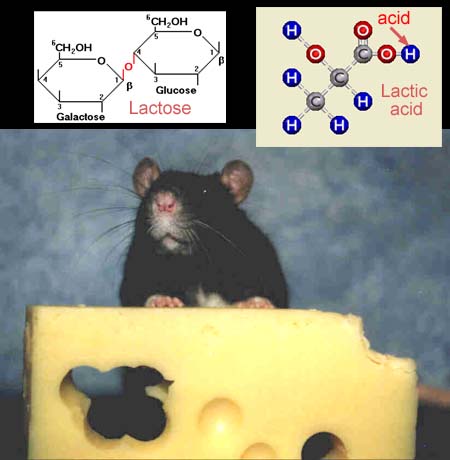
Certain bacteria and molds are used to convert milk to
cheese. At first, however, milk needs to be curdled so the solid part separates
from the liquid. This is accomplished by enzymes removed from the fourth
stomach of a calf. Some added bacteria converts the lactose sugar in the
milk to lactic acid. The lactic acid kills other microbes, which is how
cheese gets preserved. The blue hydrogen atom comes off easily and that's
the acid that kills other microbes. Molds breakdown fats and proteins. Reducing
the amount of fat helps the cheese store longer, too. We need our pottery
to keep the mouse away from our cheese. |