|
|
Organic
compounds are compounds that usually come from organisms.
They always have carbon plus a few other elements like hydrogen, oxygen,
and nitrogen.
Examples: Alcohol, Sugar, Fat, Protein.
|
|
At one time it was thought that the makeup of living things was totally different that that of non-living things. A clue for this difference was how they responded to heat. For example, think of cooking an egg. The egg white starts off clear and liquid but heating it makes it white and solid. When cooled, it doesn't return to its original conditon. However, heat up lead, for example, and it will melt, but upon cooling it returns to its original condition. So there seemed to be a difference. It was also believed that living things had a "life force" in them, which again made the chemicals in living things unique. There was a belief that the chemicals in living organisms (organic compounds) could not be synthesized by man using non-living chemicals (inorganic compounds). |
|
However, in 1828, a chemist named Friedrich Wöhler accidently created urea. Urea was a compound that mammals produced to get rid of excess nitrogen. Urea is secreted in their urine. Friedrich created it using inorganic (non-living) salts. Everyone was surprised, but chemists then knew that it was possible to create chemicals found in the body using chemicals from the ground or air (non-living sources). So now organic compounds were not defined as only those compounds from organisms, but compounds based on carbon. |
|
|
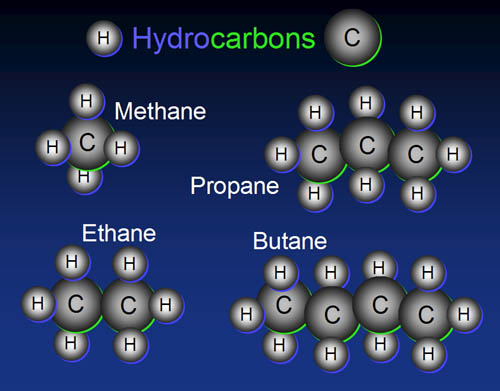
Hydrocarbons
are the simplest of the organic compounds. As the name suggests, hydrocarbons
are made from hydrogen and carbon.
The basic building block is one carbon with two hydrogens attached, except
at the ends where three hydrogens are attached. Remember carbon has vacancies
for four electrons in its outer shell. So it wants to bond to four atoms.
Here we see hydrocarbons with one to four carbons: methane (natural gas),
ethane, propane, and butane(lighter fluid). |
|
|
When the chain is between 5 and 9 carbons,
the hydrocarbon is gasoline. About a dozen carbons and it is diesel. Around
20 carbons is motor oil. A chain of hundreds to thousands of carbon and
hydrogens make plastic. This particular plastic is polyethylene. |
|
|
Before we go to
the next building block, let's look at the simplest hydrocarbon, methane,
in three different representations. The upper right shows elements as spheres
with their chemical symbol. The left one shows carbon's 4 electrons being
shared with 4 hydrogens. This is the shaped of the electron clouds. The
bottom right one helps us see carbon's four outer electrons and the four
vacancies that four hydrogen atoms could fill. Instead of four hydrogens,
what if we slipped in an oxygen? |
|
|
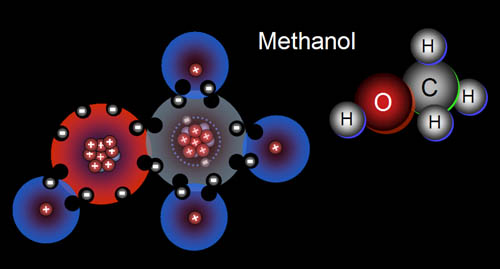
Methane becomes
methanol. Here are two representations of methanol. Oxygen is a more versatile
building block than hydrogen. Hydrogen can only connect to one other atom
because it only has one electron and one vacancy to offer. Oxygen, however
has six outer electrons, meaning it can reach its full eight electrons by
sharing one electron and one vacancy with two different atoms. Here we see
oxygen connected to both the carbon and to a hydrogen. |
|
|
By combining oxygen
with carbon and hydrogen,
we can make any kind of alcohol. Here are the
simplest. Methanol is also called wood alcohol.
Ethanol is drinking alcohol (also called grain
alcohol). Three carbon alcohol is called propanol.
If the alcohol group (OH) is on the middle carbon, it's called isopropyl
alcohol (rubbing alcohol). |
|
|
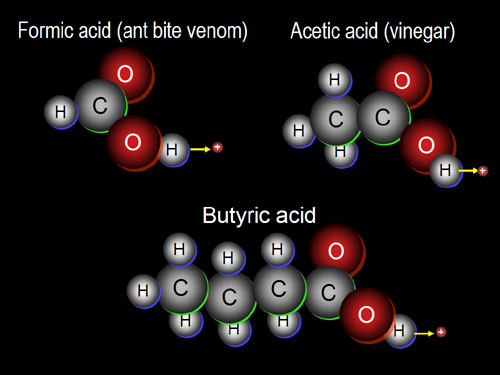
Alcohols are not
the only compounds that use the three elements of oxygen, hydrogen, and
carbon. Organic acids is another class of organic
compounds that uses these three elements. Let's start with a few common
small organic acids. You should notice that an extra oxygen
replaces two hydrogens. Remember, an oxygen has two vacancies and two electrons
to share. Formic acid is the smallest organic acid. Acetic acid is very
common. Butyric acid is produced when butter goes rancid. These are all
acids because the hydrogen bonded to the oxygen can come off easily. More
exactly, just the nucleus (1 proton) of the
hydrogen comes off. Hydrogen's electron stays behind. |
 |
Organic acids combined
with alcohols are building blocks for all
kinds of flavorings and fragrances. In this example, propanol
is boiled with acetic acid. As the bump into
each other, the OH from the acetic acid combines
with the hydrogen from the alcohol to make water,
H20.
|
|
|
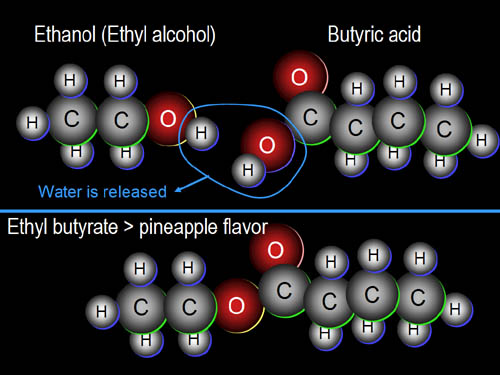
One more example
is ethanol mixed with butyric
acid. Upon boiling, they combine by releasing a water molecule. The
result is ethyl butyrate, which has a pineapple
flavor. It's interesting how flavors can come from molecules that have no
similarity with the final flavor. Stay tuned for a link that will have many
more examples. |
|
|
Another
class of compounds that use carbon, oxygen, and hydrogen is called carbohydrates,
or the slang word, "carbs." The name,
carbohydrates, is a good one because it indicates carbon
and water. Remember, dehydrated
means loss of water, and to be hydrated means
to add water. |
| Below are some examples
of carbohydrates. Sugar, starch, cellulose (wood fiber), and glycogen. Glycogen
is like starch but is created in animals for the purpose of storing chemical
energy. The small black granules (dots) are glycogen. |
|
|
|
|
With energy from light, plants can build sugars from carbon
dioxide and water. Glycerin
(also called glycerol) is not a sugar but is basically one half of a glucose
sugar. The repeating building block seen in sugars is a carbon
attached to one hydrogen and one
oxygen that is also attached to a hydrogen
(OH). |
| Below we see the sugar glucose
in different forms. The left one shows glucose as a straight chain and with
elements shown as spheres (space modeling). The next one is the same except
drawn with element symbols and lines representing bonds. In water
glucose is constantly bending and forming itself into two different ring
configurations millions of times a second. In both cases the oxygen at the
end of the straight chain attaches itself to the carbon on opposite end.
The hydrogen jumps off the oxygen and turns the double-bond oxygen (=O into
OH [hydroxide]). Sometimes this OH will end up oriented downward (alpha-glucose),
and other times the OH will be oriented upward (beta-glucose).
|
|
|
|
|
The alpha version of the ring shaped glucose
is used as building blocks to make starch.
The alpha glucoses are arranged side by side. The hydroxides (OH) from both
sides donate a hydrogen. One of the hydroxides
(OH) donate a oxygen. Water is formed and released. The left over oxygen
forms a bridge to connect the two glucose molecules. |
|
|
This picture shows the chains of alpha-glucose
in a simplified form, but you can still see the oxygen bridge and the top
carbon with its hydroxide (HOCH2). Sometimes these chains are cross-linked.
This makes a spiraling form of starch called glycogen. Animals use this
form to store energy. |
 |
The little black granules are granules of
glycogen, which again is chains of glucose. When energy is needed these
chains are broken up into glucose sugar that can be burned for calories. |
 |
Recall that when glucose bends and connects
to make a ring, it can also form the beta-glucose version where the OH is
oriented upward. Plants use this form to make celluose (fiber, plant walls,
wood). For cellulose the glucose is oriented in alternating up and down
positions as they are chained together. Again water is released as they
connect. |
 |
We should pause a moment to marvel at the
diversity that plants do with two simple molecules (CO2 and
water). With these, plants make sugars, starch, and cellulose. Consequently,
sugars are used to make oils and fats. The general name for oils and fats
are lipids. |
|
|
Lipids (oils and fats) are another class
of organic compounds built from oxygen, hydrogen, and carbon. It's amazing
what these three elements can build! |
|
|
Above, you learned a few organic acids. Organic
acids with long chains of carbon are called fatty
acids. These long "tailed" acids don't dissolve in water
and are oily. If every carbon in the chain has all the hydrogens it can
bond to, then the fatty acid is said to be saturated.
The middle fatty acid has two carbons that are one hydrogen short. That's
because there's a double bond between those carbons. This fatty acid is
said to be monosaturated. The bottom fatty
acid have two places where carbons don't have all the hydrogens. This is
polyunsaturated. The polyunsaturated fatty
acids are said to be healthier. |
| Below you see a glycerin molecule
(introduced above). When three fatty acids attach to the glycerin, we get
an oil (both vegetable and animal) and fats. Fatty acids with longer chains
create fats. Like many reactions, a water molecule is released. |
|
|
|
|
Here is the result of the three fatty acids
attached to the glycerin. Yes, it's cramped so the fatty acid chains do
spread out. Fat and oils are useful for energy storage and for building
cell membranes and fatty tissue. |
|
|
How do we make protein? Hydrogen, carbon,
and oxygen are amazing building blocks, but to make something as complicated
as protein, we need another element. That would be nitrogen. |
|
With nitrogen, we have a complete countdown
of connections-4, 3, 2, 1. This gives us more diversity with shapes. |
|
The shapes of molecules with carbon, oxygen,
and hydrogen are primarily chains, hexagons, and small tetrahedrons (3 sided
pyramids). They can make a lot of different compounds, but their shapes
are somewhat predictable and regular. To make proteins, we need much more
variety. |
| Below is a simple protein, but
notice where the yellow arrows are. One shows a six-sided ring. That is 6 carbons
forming a hexagon. Next to it are two 5 sided rings. Nitrogen is involved
in these 5 sided rings. Nitrogen contributes to diversity of protein shapes
in two ways. One is that it can make 5-sided rings to add to the diverse
shape of the molecule. (By the way, the little V-shaped rods represent water
molecules. The red portion is oxygen, and the two lavendar ends are hydrogen)
They can pass through a hole in this protein. |
|
|
 |
The other way nitrogen helps to add diversity
is its charge. But first we have to talk about amino acids. Amino
acids are the building block for proteins. The word,
amino, is from ammonia.
This is where the nitrogen comes from. The acid
part of amino acid is from the acetic
acid portion of amino acids. |
|
|
This is glycine, the simplest of the amino
acids. The amino part is the end with the nitrogen
attached to a carbon, and the acid part is
the other end where the proton from hydrogen
can come loose. Chemically, glycine is acetic acid with an amine group. |
|
|
Mammals only use 20 different
amino acids to make the immense variety of proteins it needs. These 20 different
proteins are the same as glycine above, except one of carbon's hydrogens
is replaced by some combination of the five elements on the right. Sulfur
(S) is needed for two of the 20 amino acids. The proton bound to the acid
part of the amino acid will come loose and be attracted to the top end of
nitrogen where there's a pair of electrons. This gives amino acids the ability
to push and pull on other amino acids that have charges. |
 |
Here's another protein. You may notice the
six-sided carbon rings. However, in the middle the protein is quite complex.
Again, it's the amino acids ability to have plus and minus charges that
push and pull on each other and form a almost infinite number of shapes. |
 |
Proteins are made by connecting amino acids
together. Here the acid end of glycine will connect to the amino part of
alanine. A water is released in the process. |
 |
This is after the two amino acids are connected.
Notice the ends still have one amino end (left) and an acid end (right).
That means more amino acids can be connected on either end. So the building
process continues until a protein is built. It might have hundreds or thousands
of amino acids. |
|
STRUCTURAL
CHEMICAL |
Amino acids build two types of proteins
Structural: This
type is used in building structures in an organism
Chemical: This type
gets involved in chemical reactions. Antibodies and enzymes perform chemical
chores. |
|
|
| The main building blocks of keratin mentioned above are the two simplest amino acids, glycine and alanine, which were shown earlier. Chains of amino acids like these have a tendency to form a twisted chain (helix). That forms a strong but flexible structure. |
| Below are a few examples or
chemical proteins. The protein, hemoglobin, is used because of its chemical
ability to carry oxygen. Antibodies are specialized proteins. |
|
|
|
We will end our organic building blocks
with the ultimate of building blocks, DNA.
Below is one rung in the DNA ladder. We need just one more element to
build it. That is phosphorus. Phosphorus
is surround by four oxygens (phosphate),
These form the outer rim of the helix coil. See yellow
and blue spheres in picture. The phosphate
is connected to a ribose sugar (5 carbon
sugar). This is connected to either a single ring or double ring of carbons,
nitrogens, and hydrogens. Guanine and cytosine rings are shown, but there
are two more types of rings. Guanine and Cytosine are held together by hydrogen bonding, which is where a hydrogen atom attached to either oxygen or nitrogen is attracted to a nitrogen or oxygen on the other molecule. Those are the dotted lines. These are weak bonds so they can come apart when the DNA is duplicated, yet they are stong enough to pull these two strands together into a helix. |
|
|
| Below
are two rungs of the DNA ladder. It's amazing that only five elements are
needed to make DNA. Three rungs of the DNA ladder is needed to code each
of the 20 amino acids. With millions of these rungs, you can code many amino
acids that are assembled into all the proteins a body needs for making structures
and for directing chemical reactions (enzymes, hormones, chemical proteins). |
|
|
| Below is a table that summarizes
the elements used to make the various kinds of organic compounds. Trace
amounts of iron, copper, and magnesium is used in some organic compounds.
For example, hemoglobin needs one atom of iron to help carry oxygen. Chlorophyl
needs an atom of magnesium. Other proteins need an atom of copper. The amazing
thing is the immense diversity of life done with just a few elements acting
as building blocks that build large building blocks that building even larger
building blocks. |
|
|