|
|
In the previous section we learned we could
make these three building blocks (protons, neutrons, and electrons) out
of energy. Now these three will be used to build the elements, or more specifically,
the atoms of each element. |
|
Before we get into their building block capabilities,
it's useful to know that you already have a personal connection to these
particles through your senses.
SIGHT: All light or colors that you see are the result
of movement by electrons. When electrons slow down or stop, they give
off light including visible light.
|
|
|
TASTE: You taste protons when you sense a food has a tart,
sharp, or vinegar taste. Protons are being released by acids in the food.
|
|
|
WEIGHT: You feel the weight of neutrons because
they roughly double the weight of everything you lift. |
|
Every textbook I've seen introduces protons, electrons, and neutrons as the building block of atoms, and they do it in a nonchalant, matter-of-fact manner. They also either mention or imply that all protons, electrons, and neutrons are identical to every other proton, electron, or neutron. That is true, but think how amazing that is. I'll repeat that. Every proton in the universe is exactly like every other proton in the universe. The same is true with electrons and neutrons.
How do you make things exactly alike? When we manufacture items, they may look the same, but upon close examination, there's always some differences. It's impossible for us to manufacture any two items that are exactly alike (unless we do it atom by atom). Yes, nature has produced countless quantities of these particles (protons, electrons, neutrons) that are always exact copies.
The only thing that I know that also has exact copies are numbers. For example, a "3" used here is exactly the same "3" others may use. We don't have to worry about a count of 3 that I do is different than the count of 3 that you do. So the only explanation I have for protons, electrons, and neutrons having exact copies is that they must be mathematically engineered. In other words, some kind of mathematical formula must be directing their assembly.
We have all heard that everything is unique. Even "identical" twins have differences, but the protons, electrons, and neutrons in these twins could easily be exchanged with ones from the atoms that make up the dirt or grass. Yes, the protons, electrons, and neutrons in the atoms of your body, could be exchanged with any protons, electrons, or neutrons from anything in the universe and you would never know the difference because they are identical. I find that odd and amazing at the same time. |
|
|
Let's get back to building blocks. What makes good building
blocks?
The ever popular “Tinkertoys” gives us some
clues.
Blocks must allow for ways to connect to one part to another.
Tinkertoys had circular wood blocks�with eight holes around
the perimeter and one through its center. Sticks�acted as connectors.
With sticks of different lengths and a few kinds of blocks
with holes in them, a wide variety of things could be assembled. |
|
|
How do the proton, neutron, and electron
compare as building blocks? |
|
|
Electrons by themselves would be poor building blocks
because like charges repel, and they would
just repel each other.
Protons have the same problem. They
repel each other and would build nothing.
But electrons are attracted to protons. Roll
cursor over image to see animation. |
|

|
In the above animation, you see that electrons are attracted
to protons, but the electrons don't fall completely into the proton. The
electron takes up an orbit around the electron. Part of the reason is
that electrons are not just particles, they also behave as a wave. So
the wave nature of electrons keeps them from colliding with the proton.
To see animation move cursor over the image. The
oscillation of the electron creates a spherical electron "cloud."
Here, one electron and one proton make up the element, "hydrogen." |
|
|
Let's say one electron is in orbit around
a proton. What would a second electron do? To see
animation move cursor over the image. Notice the
approaching electron is repelled by the orbiting electron, but at the
same time it is attracted to the proton. Its movement is governed
by these opposing forces. Also notice that when the second electron
joins the first, they stay as far as possible away from each other. (by
the way, electrons also spin, and the second electron has to spin [I don't
mean orbit] in the opposite direction. Spinning is not shown in this animation) |
|
|
Here are two hydrogen atoms. Notice that the electron
on the left atom is attracted to both it's own proton and to the right
proton of the right hydrogen. Likewise, the electron in the right hydrogen
is attracted to both protons. So even though protons repel each other,
the fact that they pull on electrons in the other atoms, causes them to
pull together. |
|
|
Two hydrogen atoms will stay
together because the electrons have room to stay away from each other as
they orbit both hydrogen atoms. So even though there is repulsion between
the electrons and between the protons, this arrangement minimizes the repulsions
allowing for the opposite charge attractions
to keep them together.To
see animation move cursor over the image. When two
atoms share electrons, we say they are bonded together. |
|
|
We see that the proton and electron come together to make
an hydrogen atom. We also see that two hydrogen atoms will come together
and share electrons. However, one proton has a limited attraction force.
Two or more protons together would have a greater attraction force on electrons
and give us more building block varieties. This is where the neutron comes
in. |
|
|
This is the helium atom. It has
two protons, two neutrons, and two electrons. Even though we've been saying
that like charges repel, protons very close
to each other feel a new force called the "Strong
Nuclear Force." That force causes protons to attract
each other. Being neutral, neutrons normally have no effect on protons,
but when they are very close, they attract them strongly with the same "Strong
Nuclear Force." In short, neutrons help hold
the protons together in the nucleus of the atom. |
|
|
To the left are
two fluorine atoms. The nucleus of the fluorine atom has nine protons. This
is possible because the neutrons help hold them together. The nine protons
have a tremendous pull (attraction) to electrons in the vicinity. Fluorine
has nine electrons surrounding it. Two orbit close to the nucleus. The outer
seven spread out to maximize space between them. However there is still
space for one more electron. Notice how the two electrons are being attracted
by protons from both atoms. This pulls the atoms together, and because there
is room, the atoms bond. |
|
Here the fluorine atoms have pulled together and are sharing
one electron each. They are now bonded. This is called a single
bond (even though two electrons are involved)
Atoms naturally pull on each other because the protons
in them pull on the electrons of the other atoms. However, there has to
be room for the electrons to merge and be shared by both atoms, otherwise
electron repulsion keeps them apart.
Note: Many atoms have room for eight outer electrons.
|
|
|
Here is carbon. It has six protons,
six neutrons, and six electrons. You can see that there are four outer electrons
and spaces for four more electrons. This is similar to Tinkertoys, which
had blocks that had eight holes around their perimeter. From this picture
you can see that carbon could accommodate four electrons
and share four of its electrons. Remember fluorine
above? Can carbon connect to fluorine? |
|
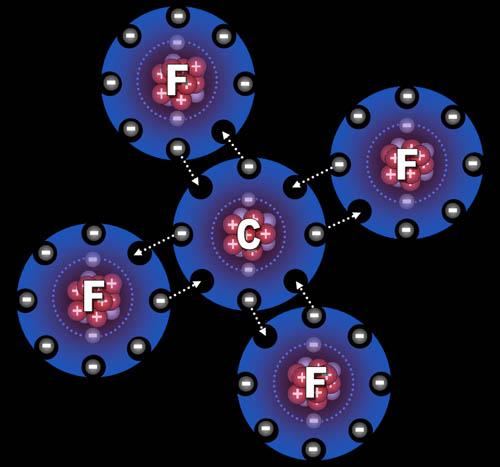
Fluorine "wants" one electron to fill up its
outer shell of electrons. Carbon will provide that electron as long as
fluorine shares one of its electrons with
carbon.
You can see how four fluorine atoms can connect to one
carbon atom. This is exactly what happens. They come together to form
carbon tetrafluoride, which is a gas used in refrigeration systems and
also fire extinguishing systems.
More importantly, you see how protons, electrons, and
neutrons make elements, which are the building blocks for what we call
"compounds." Compounds
are two or more different elements bonded together. |
|
Protons, electrons, and neutrons build elements in a rather
straight forward manner. For each additional
proton, a new element is created. For
each proton, an additional electron is attracted. The number of neutrons
also increase but not necessarily one neutron per proton. Below are the
first six elements. The orbits aren't shown. |
|
|
| Below I've added nitrogen
and oxygen, which are the 7th and 8th elements. These four are important
building blocks for living organisms. Atoms are usually drawn as spheres
to help show the space that the electrons occupy. The number next to the
element name is how many protons it has. |
|
|
| Above, we draw electrons as little
balls that orbit the nucleus, but that's really underestimating the amazing
ability of the electron. See movie below a better understanding of what
electrons can do. |
|
|
|
In the Quicktime movie above, you see the normal electrons "orbiting"
the nucleus. However, the electron forms an oscillating cloud around the
nucleus. The electron can also change shape
in order to maximize space between it and other electrons. Electrons are
always moving and their exact position is not set but based on probability.
In other words, the orbits we've been showing are their most probable
position. But there is a chance that electrons can be elsewhere at least
for a moment. There are probably several electrons in your body that may
be yards away from you at this moment. Again, electrons are amazing little "particles." If you don't see the movie, you may not have Quicktime installed on your computer. |
|
|
|
In the above Quicktime movie, you see carbon with its six electrons
orbiting it. Again, this is a simplistic view of the electrons. First,
let's change the inner two electrons to the spherical electron cloud that
better represents them. The next two electrons also occupy a spherical
shell. The next two electrons, however, form the shape of four lobes with
each electron being two lobes. Sometimes when carbon bonds with other
atoms, these four outer electrons will blend their shapes to form four
equally shaped lobes that project out in four directions. The arrangement
of the lobes match a four sided pyramid called a tetrahedron. A tetrahedron
allows the electrons maximum separation in 3 dimensions. |
| Below are four ways that we've
represented a carbon atom. The left one is drawn to show the four available
spaces for sharing electrons. The bottom left shows all the electrons orbiting
particles and contained in a sphere. The second from the right is the same
but without the spherical shell. The far right shows a more accurate shape
of the electrons. One electron is in each of the four lobes. However, two
electrons can fit in each lobe (if they spin in opposite directions). Therefore,
there can be a total of eight electrons in these four lobes (2 per lobe).
So this is similar to the left drawing that shows eight electrons would
fill the outer shell. The right one also shows the tetrahedron (4 sided
pyramid) shape that correctly shows how it will align with other atoms that
bond with it. |
|
|
 |
EXTREME DIVERSITY
Even though the building of elements is simply adding
one proton, one electron and usually one neutron for each new element,
the properties of elements can change dramatically and provide a wide
range of building blocks. |
| Electrons bend themselves into
four different shapes (also called orbitals) shown below. They are classified
as s, p, d, and f. The letters were chosen
based on the spectrum of light they emitted. For example, the s
electrons emitted sharp lines of color. d-shaped
electrons emitted diffused lines. The Periodic
Table of the Elements, shown below in a simplified manner, organizes elements
according to the number and shape of their outer electrons. The first two
columns of elements have the spherical s-orbital electrons. Column 1 has
one s electron, and column 2 has two s
electrons. Columns 3 through 12 have d-orbital
electrons where each electron usually has four lobes. On the right, the
elements with blue background (columns 13-18)
have p-orbital electrons as their outer electrons.
The p-shape is two lobes for each electron.
The bottom two green rows contain elements
that have f-orbital electrons as the outer
electrons. One element, "U," is uranium. The four types of electrons
give elements different ways of connecting. Even within one type, there
are variations because of the number of electrons. For example, the d-orbital
can have from 1 to 10 electrons. That changes the way elements combine.
In short, protons, neutrons, and electrons have built over a 100 elements
that can connect in a variety of ways. |
|
|
| Not only has protons, electrons,
and neutron built a diverse array of elements, each element can can exhibit
different properties depending on how the atoms of that element is arranged.
Take carbon, for example. Below carbon is using the tetrahedron shape, allowing
it to connect to four of its neighboring carbon atoms. This makes for a
very strong and rigid arrangement, and accounts for why diamonds are the
hardest substance known. |
|
|
| Below shows carbon arranging itself
in six-carbon rings, which form layers. This is not a strong arrangement,
but it allows the carbon layers to slide over each other. This is graphite,
which is good as a lubricant (for locks) and for pencils, so they can slide
easily. |
|
|
|
Carbon sometimes stacks itself in a random
manner. This is true of soot. The word, amorphous, means it has not regular
shape. This arrangement makes it soft and also very black, which is good
for making pigments. |
|
|
| Carbon can sometimes arrange itself
into spheres, nicknamed buckyballs. Chemists are still researching the many
possible uses of this shape. |
|
|
| A real exciting field is that
of nanotechnology, the technology a building structures at the atom level.
Below is a diagram of carbon atoms connecting in six sided rings that are
connected to make microscopic tubes. Many exotic properties are possible
with carbon in these geometric shapes. |
|
|
| In summary, protons, electrons,
and neutrons gave us a super set of building blocks called elements.
The elements now become the building blocks for compounds.
Compounds are two or more elements bonded together.
The number of compounds are almost infinite and form the incredible diversity
in the world around us. But remember, no matter how complex things appear,
there is simplicity in the building blocks that made it. |
 |
| The next
section looks at the compounds that make up everything around us. |