
A motto I came up in one tutorial was: "Things are not as complex as they look nor as simple as they look."
We can blame our senses (mostly eyes) for these two misperceptions. As mentioned before, our vision is quite limited, making some items look more complex than they really are and others look simpler than they really are.
Learning chemistry is like getting a boost to all senses, which can help us simplify what we see but also see things at a deeper level.

The movie, Matrix, had a part that really made me think of one goal of chemistry, which is to see the underlying simplicity of our world.
Even though the Matrix was just a computer simulation, humans could not help but perceive the Matrix as reality.
At one point in the movie, Neo started to reject his normal senses and started to take a hard look at what was around him.
What once seemed impossible was possible. What once seemed complex was now simple.
The world of the Matrix was not millions of different items acting in a life-like chaotic manner. No, he could see its underlying secret. All was made from the same thing, digital signals of a computer program. With this knowledge, his perceptions became accurate and he gained control of that world.
I see the task of learning chemistry very similar to Neo's challenge. We can't look at the world the same as we did before. We have to see the simplicity and complexity beyond what the eyes normally see. Only then can we see the world in a way that lets us understand and control it.
One tool for seeing our world is to see it in terms of building blocks, force & energy, and math.

Regarding building blocks, we know they build compounds that are often categorized as either inorganic or organic. Oxygen is the primary building block for most inorganic compounds. For organic compounds, carbon rules that domain.
For inorganic, let's concentrate on the 3 most abundance elements in the Earth's crust (the soil). Those are oxygen, silicon, and aluminum. For organic, let's concentrate on the carbon, hydrogen, oxygen, and nitrogen.

As mentioned, a goal I have for students in this class is to look at the world differently and to find simple connections. For example, where do you see oxygen? The sky you see and the air you breath has oxygen. Your body is 60% oxygen*. The soil underneath you is 60% oxygen*, and the grass you feel is about 60% oxygen*. Air, body, plants, and soil are all connected through this one element.
(* % is by weight)

Building Blocks: Quartz
Problem 1: Quartz is a common mineral because it contains two of the most common elements in the Earth's crust. What are those two elements?
Problem 2: What percent (by weight) of the Earth's crust are each of those two elements? (See Building Block Inorganic Compound tutorial or other sources)

Building Blocks: Glass
The main ingredients of glass are SiO2 (74% w/w), Na2CO3,
and CaO with some MgO and Al2O3 added for chemical resistance.
Problem 3: What element is in common with all of these compounds?

Building Blocks: Oxygen
Problem 4: Every year there are mine cave-ins that kill many miners due to suffocation. It always seems ironic to me because the rocks around them is about half oxygen. A 10 pound rock has about 5 lbs of oxygen in it, but it's tied up in quartz, and it's almost impossible to free the oxygen from quartz. Mines usually have water in them, and with a 9 volt battery (or higher) it's easy to split the water to get oxygen. It's called electrolysis. What is the other gas that would be produced when oxygen is being produced?
Extra Credit: If you took one liter of water and did electrolysis on it, how many grams of oxygen would you get?
Follow up Extra Credit: If a person needs about 2 grams of oxygen per minute, how many minutes of breathing will one liter of water provide?

Building Blocks:
Problem 5: When you are walking on the ground, what predominant element are you standing on?
Extra Credit: When you walk on water, what is the predominant element (by weight)? What is the predominant element (by number)?

Building Blocks: Aluminum
These gems are made from the same thing
that sandpaper is usually made from, which is corundum.
Problem 6a: What is the formula for corundum?
Problem 6b: What quality of corundum makes it good for use in sandpaper and also good as a gemstone?

Building Blocks: Silicone, Silicon, Silica
These three words are often confused. One is an element used to make computer chips, one is a compound used to make glass, and the other is used as a glue, breast implants or bigger than life molds like in this picture.
Problem 7: Which is which?
For more interesting pictures of objects from this sculptor (Ron
Mueck), go to www.flickr.com and do a search for "mueck".
Extra Credit: What are the formulas for silicone, silicon, and silica (in that order)?

Building Blocks: Hydrogen
Problem 8:
Which element is in all of these inorganic acids?
Problem 9: Gas bubbles are usually seen when a strong acid is placed on metal. What is that gas?
Extra Credit: Nitric acid added to hydrochloric acid forms a new acid. What is the name of that acid and what is the metal that it is known for dissolving?
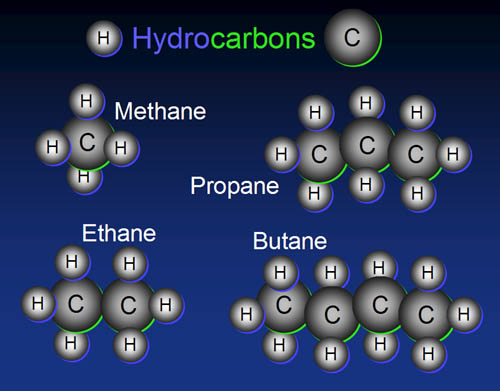
Building Blocks: Carbon
In the organic world, carbon rules. The simplest
organic compounds have just carbon and hydrogen. Carbon atom chain together
nicely to make whatever length you want from two to two million.
Problem 10: Which of these hydrocarbons is the main ingredient of natural gas?
Problem 11: Which
one is normally put into tanks for barbecues or RVs?
Problem 12: What is the name of the hydrocarbon if ethane and butane were connected?
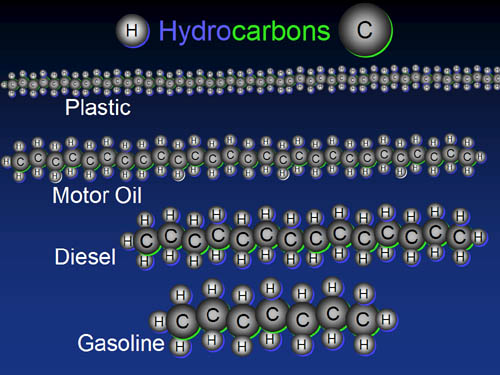
Building Blocks: Hydrocarbons
As the chains get longer, the hydrocarbons go from a gas to a liquid to a more viscous liquid, and finally a solid. The longest chains are polyethylene plastic.
Problem 13: If the
average length of a long polyethylene molecule (a common plastic) is 100,000 carbons long, how
many gasoline molecules could you make from just one polyethylene molecule?
(assume gasoline averages 8 carbons long)
It makes you wonder why all this waste plastic isn't being turned in to
gasoline. (some folks are working on it though)
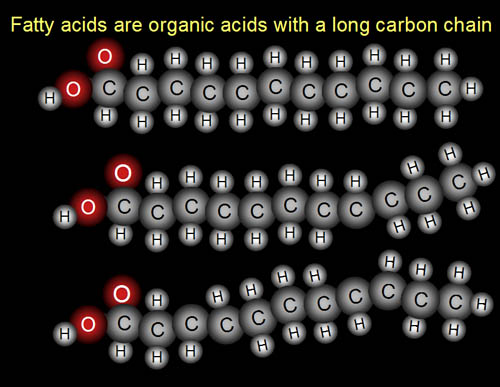
Building Blocks: C,H,O (fatty acids)
Here are three fatty acids.
Problem 14: What
part of the fatty acid molecule is the "fatty" part and which
part is the "acid" part?
Extra credit: Eicosapentaenoic acid is healthy "Omega 3" fatty acid. Omega 3 means it has a double bond (unsaturated bond) at the third carbon from the end (like the middle fatty acid in the image). This fatty acid comes from eating fish, but fish can't produce its own eicosapentaenoic acid. So where does fish get this fatty acid?

Building Blocks: (C,O,H) Carbohydrates
Problem 15a:
One of these is the building block for the other three. Which one is the
building block?
Problem 15b: What is glycogen?
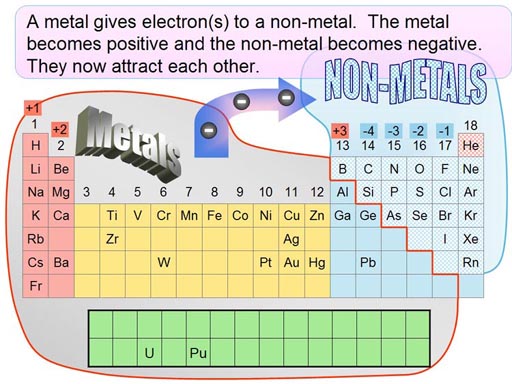
Force & Energy: Electronegativity Electronegativity is a measurement of how much an element
pulls on electrons. Elements in groups (column) 16 & 17 have the highest
electronegativity (pull the hardest on electrons). Elements in groups
1 & 2 have lowest electronegativity. So that's why they give their
electrons to those non-metals in groups 14 through 17. Salts are generally a combination of a metal in column 1 or 2 with a non-metal in column 17. The elements in column 17 are called halogens.
Problem 16: What does the "Halo" and "gen" mean in the word "halogen"?
Problem 17: The air is about 80% nitrogen with about 20% oxygen. When hydrogen burns in air, why does it combine with mostly oxygen to make H2O rather than nitrogen to make NH3? (Hint: Electronegativity of oxygen is 3.44, nitrogen is 3.04, and hydrogen's is 2.20).
Extra Credit: If the air contained 50% oxygen and 50% fluorine, what would be the most abundant compound produced when hydrogen gas is ignited? Is it H2O of HF? (Note: electronegativity of fluorine is 3.98 and oxygen's is 3.44)

Force & Energy: Problem 18: When people are in intensive care, they are often given oxygen and sugar (Dextrose). Together they provide energy because the oxygen "burns" the dextrose to get calories.
18a) Balance the equation:
C6H12O6 + O2 -> CO2 + H2O
18b) This bag is 500mL and is 5% dextrose (5g/100mL). Carbohydrates have 5 food calories per gram. How many calories are in the bag?
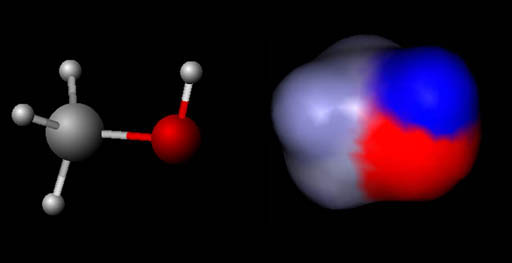
Force & Energy: Charges (methanol)
On the left is the ball-and-stick model for methanol. On the right is
a map of the same molecule but showing the charges on the methanol molecule. Blue means that region
is positive and red means the region is negative.
Problem 19:
Is methanol a polar or non-polar molecule?
Problem 20:
Which atom in methanol is in the most negative?
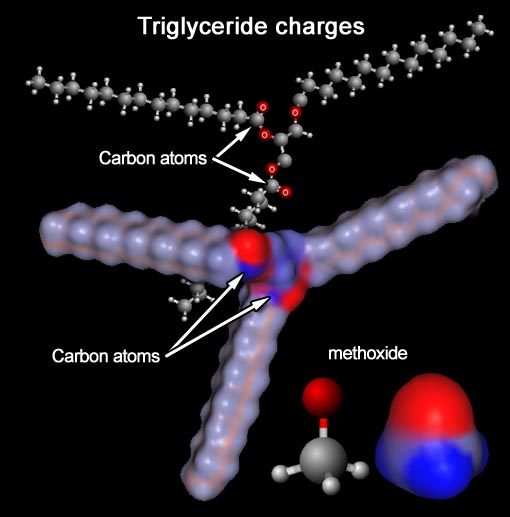
Force & Energy: Charges (triglyceride > biodiesel)
In the back is a ball-and-stick model for a triglyceride (fat or vegetable
oil). In the front is a map of the charges on the same triglyceride molecule.
Notice the arrows point to two of the three carbon atoms that sit between
two oxygen atoms.
In the front model showing charges, the arrows point to
two blue regions which are those same two
carbon atoms. In the bottom right is the methoxide ion (a negatively charged methanol). The methoxide is going to attack
those carbon atoms and break up the triglyceride and make 3 biodiesel molecules.
Problem 21: What end of methoxide is going to
be attracted to the blue carbon atoms, the oxygen end (red) or the methyl
end (carbon and 3 hydrogen atoms)?

Force & Energy: Charges
Hard water usually has calcium dissolved in it. The source is usually calcium carbonate (CaCO3) or calcium chloride (CaCl2). To the left is a molecule of soap. In water the long chain of soap has a negative one charge (-) and the sodium that was attached has a plus 1 (+) charge. Because of its charge, calcium in hard water grabs onto two soap molecules and causes them to come out of solution to form soap scum.
Problem 22a: What is the charge of calcium ions?
Problem 22b: What is the charge of carbonate ions?
Problem 22c: What is the charge on chloride ions?
Problem 23: Which would have a greater pull on the negative soap ion a sodium ion or a calcium ion?

Force & Energy: Pressure (force)
Gunpowder is made from potassium nitrate, charcoal (carbon), and sulfur.
Here's the balanced chemical equation.
10KNO3(s) + 3S(s) + 8C(s)
-> 2K2CO3(s) + 3K2SO4(s)
+ 6CO2(g) + 5N2(g).
Problem 24: When gunpowder is used to fire a cannonball, which of the products in the equation is pushing the cannonball out of the barrel?(hint: gases are responsible for making pressure)
Problem 25: What is the color of sulfur and where do you find it in nature?
Problem 26: What is the color of charcoal and how can you make it?
Problem 27: Centuries ago potassium nitrate and sulfur were used in China as medicine. It was discovered that a test that helped identify potassium nitrate was to place it in a flame. If it was potassium nitrate, it produced a purple color. Which element in potassium nitrate (KNO3) creates a purple flame? (Hint: A similar problem was in quiz from chapter 10)

Force & Energy: Energy
Problem 28: From the salad, you are acquainted with the building blocks for the elements at a personal level. Match these atomic particles to the correct sense: Neutrons, Protons, Electrons.
28a) We can taste which particle?
28b) We can see which particle?
28c) We can feel the extra weight of which particle?

Problem 29: There are many energy drinks on the market and they are packed with sugar and caffeine along with other chemicals. Surprisingly, some of these drinks claim to have no calories (no sugar or other carbohydrodrates), yet they still call themselves an energy drink. If you feel energetic after drinking one of these no calorie energy drinks and you start doing activities, where is the energy coming from?
Extra Credit: The Red Bull energy drink is banned in several countries partly because it was connected to 4 deaths even though it was not conclusive that it caused the deaths. High levels of caffeine, sugar, and taurine are its biggest criticisms. What is the chemical formula for taurine and where does it get its name?
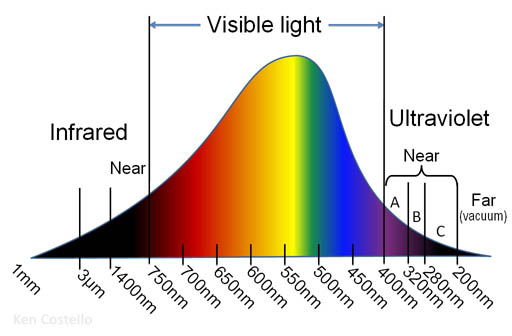
Force & Energy: Light
This is the spectrum for sunlight.
Problem 30: The sun produces much more visible light than UV light, so why do sunblock (sunscreens) advertise UV protection rather than visible light protection?
Extra credit: For UV, the graph uses the words "near" and "far". Why is it called "near" and "far"? Also, answer why "Far UV" is also called "Vacuum UV"

Math:
Earth's gravity pulls everything towards it. A helium
balloon floating in space coming under the influence of Earth's gravity
would fall towards Earth just as fast as a meteor (until it hit the atmosphere).
The only reason some items float or move upwards is because it is surrounded by a gas or liquid that is more dense than it is. In other words, a helium balloon only floats because if it is surrounded by air or liquid more dense than itself. Even a big brick made of lead will float in a bowl of mercury because mercury is more dense.

Math: The Titanic floated on water because of gravity (water being pulled down pushed the ship up) but also sank because of gravity (Titanic became more dense than water). In other words, its average density started off less than water (1 gram per mL) to something more than 1 gram per ml. Remember g per ml, g per cc, g/cc, g/cm3, and g•cm-3 are all the same.
Problem 31: Look up and report the density of solid iron in grams per cubic centimeter (or grams per ml)

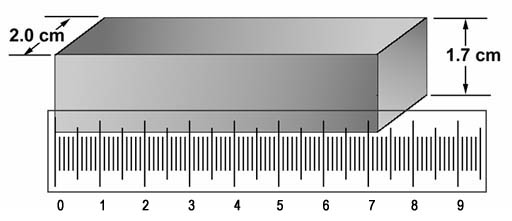
Volume =L x W x H. This bar was weighed and found to be 198 grams.
Problem 32: What is the density of iron in grams per cubic centimeter according to the picture and its weight of 198grams?
Problem 33: According to the density of the metal lithium (lookup density), would a ship made of lithium sink even if flooded with water?

Math: Calories
When you are on a diet, you are told to count fat calories,
so you look for food that is low in fat. Unfortunately, a lot of food
that is low on fat doesn't say how much fat it contains, but how fat free
it is. This container says that it is 98% fat free. At the bottom right
you see it is 6 oz. This is ambiguous. We don't know if that means fluid ounces (volume) or
weight ounces (mass). Let's say it is 6 oz. of weight.
Problem 34a: How many ounces of fat are in this container of yogurt?
Problem 34b: 1 ounce = 28.3 grams. How many grams of fat is in this container?
Problem 34c: A gram of fat is 9 calories. How many calories does the fat in this yogurt have?


Problem 36: You are stranded in the wilderness with nothing to eat and no chance of finding food, but water is plentiful. Which one of these guys would you rather have with you if it will be about a month before you are rescued and why?
Problem 37a: The picture on the left is Eric Chopin when he weighed 410 lbs. The right image has him at 196 lbs. If the weight was all fat, how many calories from fat did he burn up?
Problem 37b: A typical fat molecule has this formula C48H100O6. Balance the formula for the burning of fat.
C57H100O6+ O2 --> CO2 + H2O
Extra credit: Eric lost 214 pounds of fat with most of it going out the lungs as CO2 and H2O. If all came out the lungs, what was the total pounds of CO2 and H2O that was exhaled due to the burning of the fat?

Problem 38: My mother takes albuterol treatments everyday. She had pneumonia awhile back and the albuterol opens the bronchial passageways to help breathing. She is supposed to take 20 milligrams of albuterol sulfate for each breathing treatment.
38a) How many milliliters is that?
38b) How many treatments can she get from this bottle?

Math: Concentration
Problem 39: A patient is in cardiac arrest and you were sent to get a the injection vial for 8.4% w/v sodium bicarbonate. You found this vial. Is it the right one? (Hint: 8.4% means 8.4 g/100mL)
Problem 40: The doctor injects 10cc (10mL) of this solution. How many milligrams of sodium bicarbonate was injected?
Extra credit: The side of the label says, "nonpyrogenic". What does that mean?
Math:
Here's a bottle of Bacardi 151. Notice the flammable warning.
The top of the bottle as a metal flame arrestor (probably a wire mesh).
This prevents a flame from going down into the bottle. (Roll cursor over image to see its flammability)
Problem 41: What percent of alcohol is 151 proof?
Extra credit: Your friends are smokers and you don't want them to catch their drink on fire, so how much water do you need to add to 237 mL(1 cup) of Bacardi 151 so that it is 99 proof? (Alcohol does not burn under 100 proof) Give answer in liters or milliliters.

Math:
While we are on the subject of alcohol, here's something
even stronger than Bacardi 151. Notice all the flammable warnings and the proof and percent alcohol.
Here's the unbalanced combustion equation for ethanol:
CH3CH2OH + O2 -> CO2 + H2O
Ethanol could also be written as C2H6O.
Problem 42: Write the balanced equation
Problem 43: Liquor bottles come in sizes of quarts, liters, and fifths.
43a) Which one has the largest volume?
43b) Which one has the smallest volume?

Math: pH
Problem 44: Teeth begins to dissolve at pH 5. What is the moles per liter of H+ ions at pH5?
Problem 45: Orange juice has a pH of about 4. Sipping on it would dissolve your teeth. You want to dilute one liter of orange juice with water so that it would have a pH of 6. That way you could sip on it without dissolving your teeth. How much water would you have to add to the 1 liter to make it pH of 6.
Extra credit: The rainbow-like colors shown are those of red cabbage extra at the different pH levels shown at the top. What pigment in red cabbage is causing that color change?

Problem 46: People who have eaten bagels with poppy seeds have been fired because a drug test showed consumption of morphine or heroin. True or false?

People are always wanting to save money on gasoline. So there are always products for sale that make claims that their product will give your vehicle better gas mileage. Some of these create a gas from water that they call HHO gas (Note: This is a name they made up). A Google search for HHO gas will reveal 80,000 hits. Dozens of vendors are selling devices that produce HHO gas. It uses electricity from the alternator to split water (electrolysis) into two gases that is piped into the air intake of the car. Problem 47: What are those two gases?
These gases will give the car more power, but all vendors neglect to say that the alternator becomes harder to turn when it produces the extra electricity needed to split water. So the extra mileage is cancelled out by the extra gas the engine uses to spin an alternator that is dragging down the engine because of the heavy electrical load.

Scientific Skepticism
Here is an ice cream that is pushing its Carb Smart ingredients. With
5 grams of sugar, it's about 1/4 of the normal ice cream bar and only has
3% of the recommended Daily Value. So a person could eat 33 of these ice
cream bars and still be under 100% of the Daily Value for carbohydrates.
Problem 48a: How many of the ice cream bars will put you over the 100% Daily Value of Saturated Fat?
Problem 48b: How many bars will put you over 100% of the "Total Fat" Daily Value?
(Carb smart but not fat smart)
As a consumer, you always have to be on guard for bogus products, and believe me there are thousands of them. Here are some clues that something is wrong:
A. Technobabble (technical descriptions that are meaningless and have no basis in real science). So if you read it and it doesn't make sense, it's not your fault. It's just technobabble.
B. Fantastic claims
C. Scare tactics
Problem 49: Visit this site (link on left) and look over its different Web pages and report 3 examples of "technobabble", 3 examples of "fantastic claims" and 3 examples of "scare tactics."

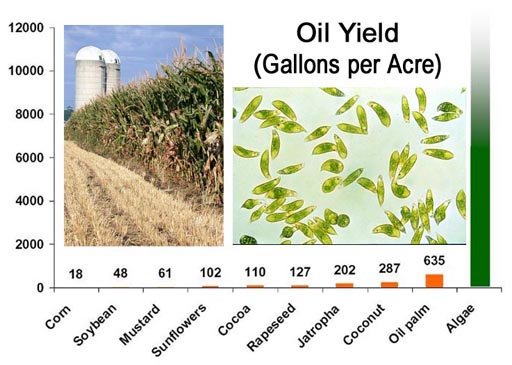
ASU researchers Qiang Hu and Milton Sommerfeld are shedding a whole new light on the algae in the Laboratory for Algae Research & Biotechnology (LARB) at the Polytechnic campus.
I got a tour of the lab last April and have been keeping tabs on this very promising resource for biodiesel, bio-jet fuel, bio-gasoline, alcohol, and food.

Math:
Here is an aerial view
(Google Maps) of where I live. The green areas are places on the roof
and in the backyard where I could grow algae. I estimate about 1800 sq.
feet is available.
Problem 50: If one acre is 43560 sq. feet, how many gallons of oil could I produce in a year if my yield is 8,000 gallons per acre?

Extra Credit: Let’s say you are kidnapped by terrorists. To prove your goodness you say you will consume three poisons: pool acid, anthrax, and lye. First you pour 250 mL of concentrated 10 molar hydrochloric acid (pool acid) into a bottle of anthrax. Then you dissolve 100 grams of lye (NaOH) in 250 mL of water. This is poured into the acid and anthrax mixture. The mixture gets quite hot but after it cools you take a drink and nothing bad happens. Why? (be specific)
Extra Credit: On the Survivorman TV show, the host will sometimes use glycerin and potassium permanganate to start a fire. In the image on the left there's a litter glycerin placed on top of the dark purple potassium permanganate. Roll cursor over image to see the fire it creates. In less than a minute the mixture will self-ignite. Here's the formula of the reaction:
14KMnO4 + 4C3H5(OH)3 --> 7K2CO3 + 7Mn2O3 + 5CO2 +16H2O
Part 1) Where can you buy potassium permanganate and glycerin? (Give me specific websites).
Part 2) In a survival situation, potassium permanganate has some other uses. What are they? (Name at least two)
Congratulations on getting through the written final. Send your answers to chm130@chemistryland.com. Use subject line of "Written Final"



