|
Oral Exam: Pronunciation of Chemical
Names and Declaration of Certain Fundamentals
|
|
|
Here I am talking to two of the four authors of the textbook
I use in a different class. When you talk to others in the field, you
hope your pronunciation of chemical names is correct.
Because this is an online class, you haven't got to hear
the pronunciation of chemical names. In future classes, I will record
these names and have them on the Web.
|
 |
Learning chemical names from a textbook is OK, but when
you walk into a chemistry stockroom, it looks very different than what
you see in a textbook. It's also a bit intimidating. So instead of just
learning the words written on a textbook page or even Web page, I like
to show the words on the containers where the chemicals are stored.
For the oral exam, I will go over the words with you to
make sure you can pronounce them. However, here I just don't want to give
a long list of names without connecting them to the actual chemicals.
So the below images are to show names along with their containers.
|
|
|
Strong inorganic acids come in these glass 2.5 liter jugs.
Glass is resistant to most acids (all but HF).
Know how to pronounce:
Sulfuric Acid (battery acid)
Sulfurous Acid
Hydrochloric Acid (also called muriatic acid or pool acid)
Nitric Acid (night trick)
Phosphoric acid
Iodic Acid (HIO3) (I oh dik)
Periodic Acid (HIO4) (Purr I oh dik)
|
|
|
99% acetic acid also comes in these 2.5 liter jugs. This
has the name, Acetic Acid, Glacial. Pure acetic acid will freeze at 62°F,
so in a cold room, it looks like it has ice crystals in it (hence the
name glacial). But those crystals are not ice, but frozen acetic acid.
This is an organic acid. Other organic acids are:
Oxalic acid (remember Dumb Cane?)
Citric acid (from citrus)
Lactic acid (from milk and in muscles)
|
 |
This big bucket contains partially neutralized phthalic
acid. The "ph" is normally silent, so you hear "thalic",
but it is acceptable to include an "f" sound before the "th",
so it could be pronounced as "fthalic acid"
The two hydrogens on the upper molecule are the acidic
hydrogens (they come off). One of these hydrogens can be replaced with
potassium. Probably KOH (potassium hydroxide) was added to the phthalic
acid and the H+ of phthalic acid combined with OH- from KOH to make water.
The potassium ion (K+) took the place of H+.
The name of this chemical is potassium hydrogen phthalate.
Remember, when an organic acid loses its hydrogen (H+), the "ic" of the name becomes
"ate".
In potassium hydrogen phthalate, the word "hydrogen"
is referring to the hydrogen that is still attached. The short name you
will hear for this compound is KHP.
|
 |
These two bottles show the name aluminum chloride prominently.
As you check closer you realize that 6 water molecules is attached to
each AlCl3. So the proper name is aluminum chloride hexahydrate.
Many salts (metal + non-metal) have a certain number of
water molecules attached. So it's important to say that along with the
name otherwise, there could be some serious mistakes because one mole
of aluminum chloride anhydrous (no water) weighs a lot less than that
of aluminum chloride hexahydrate. So amounts could be wrong and their
chemical behavior can be quite different.
|
 |
These two glass jars have some
poisonous compounds. Compare sodium arsenate to sodium arsenite. Note the
"ate" and "ite" is referring to the oxygens. They don't
actually count them, but "ate" always has more oxygens than the
"ite". |
 |
Some element names are similar
so be sure you say the right one. Here is magnesium acetate and manganese
acetate. The left one has 4 water molecules attached, so the correct name
is magnesium acetate tetrahydrate. |
|
|
Here are bottles of mercurous chloride and mercuric iodide
"red". These are the old names. The "ous" and "ic"
are no longer preferred but you still hear them.
The new names would be mercury (I) chloride and mercury
(II) iodide.
Some salts will have different colors depending on the
crystalline structure. So they might have the same formula but the atoms
are stacked differently giving them different properties and sometimes
different colors.
|
 |
Three bottles that have potassium salts.
Potassium carbonate anhydrous, potassium chloride, and potassium chlorate.
Notice the "Oxidizer" warning on the chlorate. Oxidizers like chlorates can release oxygen upon
heating and cause things to burn violently. |
|
|
Cupric acetate monohydrate is the older name. The new
name is copper (II) acetate monohydrate. Actually, if you look close,
you will see the new name under the old one.
Like many copper compounds, they are blue.
|
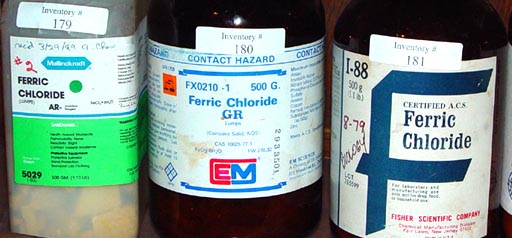 |
Ferric chloride in three different containers. The new
name is Iron (III) chloride.
|
|
|
Calcium chloride comes in various containers as well.
Notice some say "anhydrous".
The word "anhydrous" is often said after or
before the name. So you could either say anhydrous calcium chloride or
calcium chloride anhydrous. Either way, anhydrous means "no water". So no water is attached to these salts.
|
|
|
Chromates and Dichromates are usually colored. The word
"chrom" means color, so it's no big surprise. When you see oxygens
in the formula and "oxidizer" warning, that means the oxygens
can come off to create a fire hazard.
Here we have potassium dichromate.
F.W. stands for formula weight, which also gets called
molecular weight or molar mass. Just add up the atomic weights of Kx2,
Crx2, and Ox7.
|
|
|
Like I said chromates are colorful.
Here's sodium chromate. |
 |
Interestingly, a stockroom my
have chemicals that you can get from the grocery store. Here we have sodium
bicarbonate (NaHCO3) from a chemical supplier and from the grocery store
(baking soda). The chemical supplier is more apt to list the % purity and
any trace impurities whereas the grocery store version won't. If this is
used to neutralize an acid spill, it's cheaper to use the store bought box
of baking soda rather than the expensive bottle from the chemical supplier.
Sodium bicarbonate has a new name of sodium hydrogen carbonate (NaHCO3)
and you probably can see why. |
 |
Here's sodium phosphate,
tribasic (Na3PO4). Below that name is another name of trisodium phosphate
dodecahydrate. Since phosphate is negative 3 charge, saying sodium phosphate
is enough, but it's common to have one, two, or three sodium atoms on the
phosphate, so they usually indicate that as monosodium phosphate (NaH2PO4),
disodium phosphate (Na2HPO4), and trisodium phosphate (Na3PO4). This last
one is sold in places like Home Depot as a cleaner for paint preparation
and is called TSP for obvious reasons. |
|
|
Polyatomic ions make up a lot of the nomenclature. Let's
make sure you know how to pronounce them:
Sulfite (preservatives)
Sulfate (calcium sulfate using in making casts)
Hypochlorite (sodium hypochlorite used in bleach)
Chlorite
Chlorate (potassium chlorate in fireworks)
Perchlorate (also in fireworks)
Replace "chlor" above with "iod" for iodine
series
(hypoiodite, iodite, iodate, periodate)
Replace "chlor" above with "fluor" for fluorine series
(hypofluorite, fluorite, fluorate, perfluorate)
Nitrite (made by bacteria)
Nitrate (in fertilizers and explosives)
Carbonate (calcium carbonate in chalk)
Bicarbonate (also called hydrogen carbonate)
Cyanide
Chromate (colorful pigments in paints)
Dichromate
|
|
|
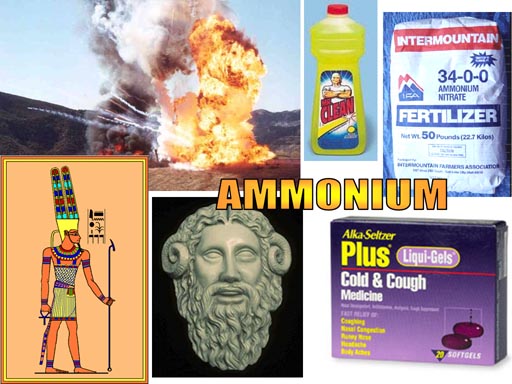
The ammonium polyatomic ion takes the place of metals in many
compounds.
Ammonium nitrate
Ammonium chloride
Ammonium carbonate
Ammonium hydroxide
|
|
|
For naming numbers Greek words are often used.
0.5 = hemi
1= mono (if mono isn't mentioned then 1 is understood)
2 = di
3 = tri
4 = tetra
5 = penta
6 = hexa
7 = hepta
8 = octa
9 = nona (Know nuh)
10 = deca (deck uh)
11 = undeca (oon deck uh)
12 = dodeca (dough deck uh)
|
|
|
Learning the names of toxic gases is good to know.
Note that the gases, HF, HCl, H2S, and HCN, change to
acids when they hit water. Their names also change:
hydrogen fluoride gas> hydrofluoric acid in water
hydrogen chloride gas>
hydrochloric acid in water
hydrogen sulfide gas>
hydrosulfic acid in water
hydrogen cyanide gas> hydrocyanuric
acid in water.
When ammonia hits water, it forms ammonium hydroxide (NH4OH).
|
 |
Here are gases from the textbook nomenclature
assignment.
a) carbon monoxide
b) sulfur trioxide
c) carbon tetrabromide
d) phosphorus trichloride
e) nitrogen dioxide
f) dinitrogen pentoxide
g) iodine monobromide
h) silicon tetrachloride
i) phosphorus pentaiodide
j) diboron trioxide
|
| |
|
| Here is the list of chemical names mentioned
above but in list form plus a few more. When we do the oral exam, just read
them to me. If you mispronounce any, I can correct you, and we can continue.
At the bottom of this page is a link to a text document that you can print out. |
ACIDS:
Sulfuric Acid
Hydrochloric Acid
Nitric Acid
Phosphoric acid
Iodic Acid
Periodic Acid
Glacial Acetic Acid
Citric acid
Lactic acid
|
SALTS:
Magnesium acetate tetrahydrate
(What does "tetrahydrate" mean?)
Copper (II) acetate monohydrate|
Anhydrous Calcium chloride
(What does "anhydrous mean?)
Potassium hydrogen phthalate
|
SALTS:
Mercury (I) chloride
*Mercurous chloride
Iron (III) chloride
*Ferric chloride
Sodium arsenate
|
Greek Prefixes
0.5 = hemi
1= mono
2 = di
3 = tri
4 = tetra
5 = penta
6 = hexa
7 = hepta
8 = octa
9 = nona
10 = deca
|
Polyatomic ions:
Sulfite
Sulfate
Hypochlorite
Perchlorate
Hypoiodite
Periodate
|
Nitrite
Nitrate
Carbonate
Bicarbonate
Cyanide
Ammonium
Gases:
Carbon monoxide
Sulfur trioxide
Carbon tetrachloride
Phosphorus pentaiodide
|
Elements:
Technetium (tek nee shium)
Molybdenum
Magnesium
Manganese
Xenon
Cesium
Neodymium
Deuterium
Tritium
|
Miscellaneous:
Alkali Metals
Halogens
Phenolphthalein
Nucleus, Nuclei
Gamma rays
Electromagnetic spectrum
D-Xylose (a sugar) (Dee zy lose)
Dextrose
Fructose (Frook tose)
Avogadro's number (a mole)
|
Radiopharmacology
Antoine Lavoisier (father of chemistry)
Metric prefixes:
deci, centi, milli, micro, nano, pico
kilo, mega, giga, tera
|
Fundamentals of Chemistry |
|
Let's begin with where the word "Chemistry" comes from. It came from the word, alchemy, which came from the Arabic word, al-kemia. "Al" means "The" and "kemia" means the black lands of Egypt (rich lands along the Nile). Egyptians were obsessed with eternal life and when they heard about Aristotle's fifth element which could turn metals into gold and be a fountain of youth, they pursued that quest. So the art of alchemy was named after the region they lived in (the al-kemia=black lands of Egypt). Also, the Greek word, khymeia, meant the medicinal use of plant extracts. So both words reinforced the idea that alchemy was an activity to find cures or create riches by investigating the substances around us. Even modern chemistry has those goals. |
|
All chemistry concepts can be broken down into three areas of focus: Building Blocks, Force/Energy, and Mathematics.
|
 |
Chemistry shows how simple building blocks build more complex building blocks, which then build even more complex building blocks. It's an efficient and flexible way to make a universe. (It's also good for any kind of building project.)
For example, atoms are made from 3 subatomic particles: Protons, electrons, and neutrons, and all elements are made from a certain number of these 3 particles.
|
|
Atoms combine to make molecules.
Molecules combine to make macromolecules. Examples would be amino acids making proteins, sugar making starches, ethylene gas making polyethylene plastic, and nucleic acid making DNA. Also small ionic molecules will grow to large crystalline structures such jewels, ores, and salt crystals.
Molecules combine to make macromolecules. Examples would be amino acids making proteins, sugar making starches, ethylene gas making polyethylene plastic (like in image on the right), and nucleic acids making DNA. Also small ionic molecules will grow to large crystalline structures such jewels, ores, and salt crystals. Macromolecules make living things (For example, proteins make muscle, skin, eyes, etc.) |
Building Blocks of chemistry also fall into two categories. |
|
Organic chemistry: Carbon rules because it is required in all organic compounds. This is the chemistry of living things (organisms). The building blocks are carbon and often hydrogen, nitrogen, oxygen, sulfur, and phosphorus (6 elements). These elements bond by sharing their electrons (covalent bonding). |
|
Inorganic chemistry: Oxygen rules not because oxygen is required but is usually present. The building blocks are usually a metal combined with a non-metal. Oxygen is often that non-metal. These elements bond because the non-metal has ripped the electron off of the metal. They then have opposite charges that attract (ionic bonding). Inorganic chemistry is basically for non-living things like jewels, ores, salts, rocks, strong acids, metals, water, and gases. |
| |
|
Force & Energy |
|
Atoms build molecules because of electrical attraction and repulsion. That's because opposite charges attract and like charges repel. The attraction between protons and electrons creates an attraction between atoms. With only attraction, atoms would all pull together to form one huge atom. Fortunately, like charges repel, so electrons repel electrons and protons repel protons so atoms only get so close. This allows atoms to build molecules and gives us the world around us. This same force causes oxygen to cling to red blood cells and all cells to carry out the process of living. To understand the body, understand how chemicals in the body either attract or repel each other.
|
|
Once atoms come together to make a molecule, it takes energy to make them come apart to form new molecules. Without energy, compounds would form but never change. So we would have a world that would end up complex but also static (no decay and no growth). However, with energy we get rearrangement and change and therefore life. Ultimately this energy comes from the sun. In photosynthesis light from the sun pulls apart carbon dioxide and water molecules and rearranges them as a sugar and oxygen. Sugar and oxygen provide the energy to form new molecules within the plant or within animals that eat the plant. So life goes on. This sugar and oxygen also gives us the petroleum, coal, and natural gas that we use. So our machines keep going too. |
 |
Mathematics is the third area of focus.
Math is used for precise measurements, but its significance goes much deeper. The physical universe has a mathematical foundation. Elements are built using simple mathematical rules (quantum numbers, chapter 10). Force and energy also follow strict mathematical rules. So even things like dirt, air, bacteria, digestion, and fire are all obeying rules of math. Math also gives us predictability. For example, we know if we add 3+2+4, we always get 9. Since atoms are mathematical, we know if we add oxygen to hydrogen we get water. Medicine is predictable because the chemicals in drugs and our bodies are made from atoms that follow mathematical rules. |
Chemistry Words of Wisdom |
|
In my studies of chemistry and science, I came to the conclusion that "Nothing is as complex as it looks or as simple as it looks." For example, diamonds, soot, copier machine toner, pencil lead, and graphite lubricant all seem very different. However, you learn that they all are made from just carbon atoms, only arranged differently. So their makeup was much simpler than first assumed. In contrast, let's look at water. You learn the formula is H2O. Simple enough. Then you learn there are 3 main isotopes of hydrogen in water. One is tritium, which is radioactive. Even though it's just a trace, you drink about a billion of them in a glass of water. You also learn there's 3 isotopes of oxygen. So that means there are 27 different waters in water. 3 isotopes of hydrogen for the first hydrogen, 3 for the second hydrogen, and 3 isotopes of oxygen. 3x3x3=27 combinations. Then you learn that the oxygen in water is created only in huge stars when the temperature is one billion degrees and carbon and helium are used as nuclear fuel. Then you learn that water is repelled by strong magnetic forces allowing our bodies to become weightless in a strong magnetic field. Then we learn that water under pressure can be turned to ice even when it over 200 degrees. So the surprises go on and on as we learn more about what appeared to be simple. But as we learn more we also see more underlying connections to things that make things simpler. So again, "Nothing is a complex as it looks or as simple as it looks." |
 |
Here's another chemistry related truism. The difference between trash and treasure is just the arrangement of the atoms. Everything we would like to own is already around us. It's just not in the arrangement we want. You want a diamond? Take the carbon out of a scrap of cloth and stack them like 3 sided pyramids and you got a diamond. You want something sweet? Take another scrap of cloth and break down the cotton fibers into the glucose building blocks that built the cellulose. Do some more rearrangement of the carbon, hydrogen, and oxygen atoms in the fibers plus some nitrogen in the air to make chocolate. You want a new car? Go dig a hole in your backyard about one yard wide, 3 yards long, and two feet deep. The dirt, grass, bugs, rocks, soil, and other stuff in the pile of dirt have all the atoms you need to make your new car. It's just a matter of rearranging the atoms in the dirt into the parts of the car. The grass and bugs will make the plastic, upholstery, and tires. With more grass you can have a carbon fiber body like race cars. The dirt also has the aluminum, iron, chrome, and copper that you need. Also present is silicon and oxygen to make the windows and electronics. The only thing keeping us from doing that is the energy to pull apart the molecules to get the individual atoms followed by a way to reassemble them and move them to where we want them to end up. Mining and manufacturing does accomplish this but not as easy as is theoretically possible. |
|
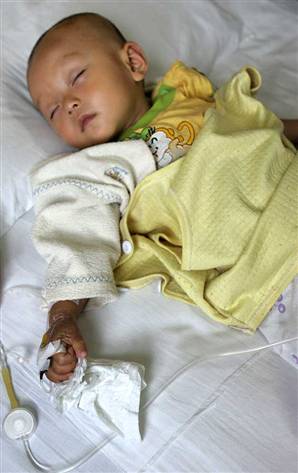
This is similar to the above truism. The difference between health and sickness is the arrangement of the atoms. In other words, the body is sick or dies if atoms are not where they are suppose to be, or there are atoms present that are not suppose to be there. For example, in the picture is a child suffering from lead poisoning. Treatment is to remove those lead atoms using chemicals that trap lead, or to give nutrients that block the absorption of lead. Lead interferes with enzymes, so ideally, treatment also needs to repair the molecules that had their atoms scrampled due to the presence of lead. |
| The text files contain a list of the words to pronounce plus it condenses the chemistry fundamentals mentioned above. Besides pronouncing the words, I will have you recite the chemistry fundamentals. There are also some questions mixed in that you need to answer. |
The oral exam is done over the phone. When you do the oral exam, you need a print out of one of the files below or have it up on the computer.
Word Format: Words to Pronounce.doc
RTF Format: Words to Pronounce.rtf
Send me an email with your phone number and a day and time that is good for me to call you. I estimate about 20 minutes to do the oral exam. We can schedule this even before you finish all the other assignments. Email me at chm130@chemistryland.com
|