Solubility is a measure of how much a gas, liquid, or
solid becomes dissolved in a liquid.
The lava lamp is a good icon for solubility. It took them years to develop formulas for the globs and the liquid, so the globs would not dissolve in the liquid but be almost the same density.

If you buy gas, you've probably seen the additive MTBE: Methyl Tertiary Butyl Ether. The oxygen in the molecule helps gasoline burn more completely. It is very soluble in gasoline. Unfortunately, it's also partially soluble in water. 42 g dissolve in a liter. It can be tasted at a very low 0.0001 g / liter, which means it would not take much to make drinking water in wells or aquifer undrinkable.
Over 20,000 of these storage tanks are estimated to be leaking in the state of Virginia alone. Again the problem comes from the fact that it is soluble in water.
Applications of Solubility Knowledge
Cleaning
Separation (purifying)
Identification
Understanding solubility will help you do cleaning because you can make an educated guess of what dissolves what.
By dissolving one substance and not another, you can separate two substances in a mixture.
Knowing what dissolves and what doesn't can be used to identify an unknown substance.


Water is known as the universal solvent. The reason is has that power to dissolve is because of its charge. The oxygen side of water is negatively charged because the protons in oxygen's nucleus pulls the shared electrons closer to the oxygen atom.
The protons in the two hydrogen atoms are then more exposed. So the hydrogen side of water is positively charged.
Since unlike charges attract, the negative end of water will be attracted to the positive sodium ion. The positive end of water will be attracted to the negative chloride ion.
Since water is always in motion, it will pull on the ionic compound and move the ions away from each other. This dissolves the ionic compound. (Roll cursor over image to see animation).

Water is always trying to pull itself into a tight ball as long as there is nothing nearby that has a charge on it. Therefore, this surface is not repelling water; it’s simply not attracting it and keeping water from doing what it does naturally.
Again, wax on a car's paint doesn't repel water, it simply
isn't attracting it. Water pulls itself into beads on
its own.


Many plants have a waxy coating on their leaves. Wax is
long chains of carbon and hydrogen that have no charge on them. So water
isn't attracted to the wax, allowing water to pull itself into beads.
This same attraction forms a "skin" on the water as the water
molecule interlock with each other. This "skin"
is also called surface tension. Some insects
take advantage of the surface tension and are able to walk on the surface
of the water.

Water molecules pull on each other so strongly they bunch up into spheres. Because of this, we are able to witness rain and raindrops.
There's probably no other liquid that can fall the height of clouds and reach the ground as drops. Most liquids would break up into an aerosol or fog.
So without water's built-in + and - charge, there would be no rain, just fog.
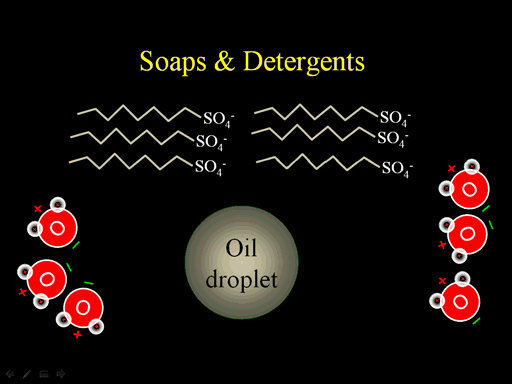
Soaps and detergents are chains that have one end that is like oil, which has no charge, and the other end is charged. Sulfate is commonly attached to the end of those long carbon/hydrogen chains (zig-zag lines).
The long chains will mix with the oil because there's a slight attraction between the long chains in the soaps with the long chains in the oil. Water will be strongly attracted to the charged ends. Once the soap is attached to the oil droplet, it will dissolve in water because water surrounds it as it holds on to the sulfate ends (Roll cursor over image to see animation. Realize they wouldn't move one by one, but all at the same time).
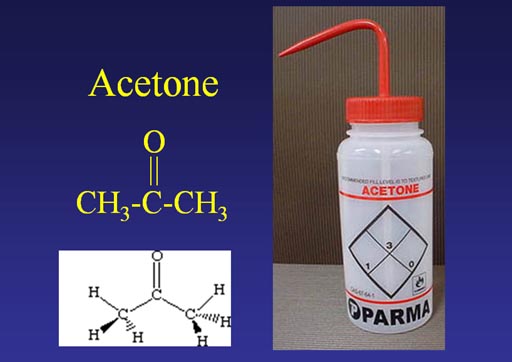
Water is called the universal solvent because it is everywhere; however, it doesn't dissolve substances that have no charge on it like wax, oil, or fat. Acetone, however, can dissolve substances that have a charge and those that don't. So it's a good all around solvent. The drawback? It's more expensive than water and it easily catches on fire.
Measuring the concentration
of a solution
A solution consists of something dissolved in water or another solvent.
A solution consists of something dissolved in water or another solvent.

There are two ways we talk about the concentration of a solution. One is an approximation of the concentration and the other gives numbers.
A solution contains the solvent and a substance dissolved in that solvent. That substance that got dissolved is called the solute.


Salt (NaCl) is very soluble in water; 350g per liter. However, if water evaporates, there will be too much salt for the water to hold in solution. The salt begins to form crystals.
A lake near Death Valley (near town of Trona) is supersaturated with salt causing the salt to crystallize out.

The salt looks like snow from a distance.
The instructors at Mesa Community College usually take their geology club students to Trona to collect salt crystals from this lake.

Here's an example of the salt (NaCl) crystals collected. They call these halide crystals.
The red color comes from a red algae that somehow is able to grow in this supersaturated solution of salt.

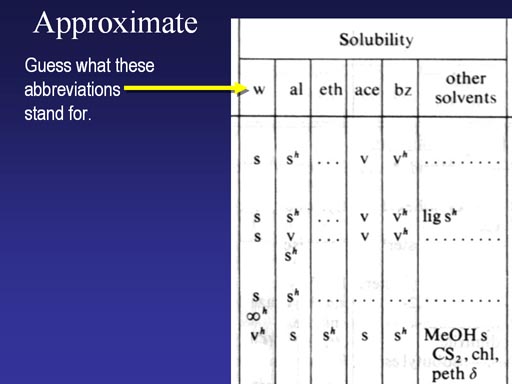
Here's a scan of one of its pages showing solubility. What do you think w, al, eth, ace, and bz stand for?
(Roll cursor over image to see answers).
By the way The "s" means soluble, the sh means soluble if the solvent is hot. The "v" means very soluble.


So far we've mentioned the ways we approximate concentration. Now let's look at quantitative ways of measuring concentration.
Here's the list but let us go through them one by one.

Mass (weight) per Volume (w/v)
Here's another segment from the CRC handbook. This is the entry for sodium
phosphide (Na3P). The heading says the solubility is in grams
per 100cc (that's a weight to volume or w/v). The solubility of Na3P
in cold water (0°C) is 5.41 grams per 100cc and in hot water (100°C)
is 93.11g/100cc. This shows you that heating up water really makes things
dissolve more. If these values are grams per 100cc, you can easily change
these values to 5.41% w/v and 93.11% w/v. This is the Mass/Volume Percent
format. So "% w/v" means grams per 100cc (or 100mL)


Here we have Crest toothpaste. They list two concentrations. One is for sodium fluoride (0.243% w/v and one is for just the fluoride ion (0.15% w/v). So that translates to 0.243 grams of NaF per 100mL of toothpaste and 0.15 grams of F- per 100mL of toothpaste.
It's nice that they both give the concentration of the fluoride ion, because you can compare the two. It wouldn't help a as much if one gave the concentration of NaF and the other Na2PO3F.

Insecticides are often dissolved in solvents (other than
water). Their concentrations are usually given as the active ingredient
weight divided by solvent weight, then converted to percent.
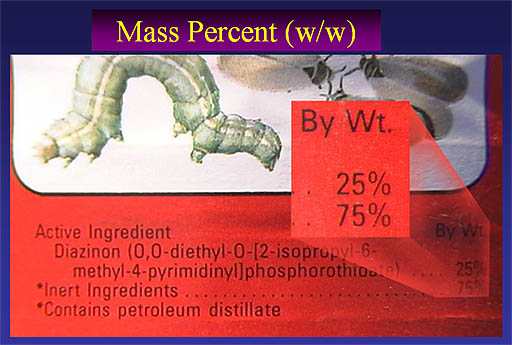
Here's a close up of the ingredients shown on the bottle above. Notice they call it "By Wt." which of course is "by weight". The active ingredient is 25% by weight, which means that whatever weight you measure, the active ingredient is 25% of that weight. So one pound of insecticide solution contains 1/4 pound of the active ingredient (Diazinon). So, again, this is a weight over weight percentage (% w/w)
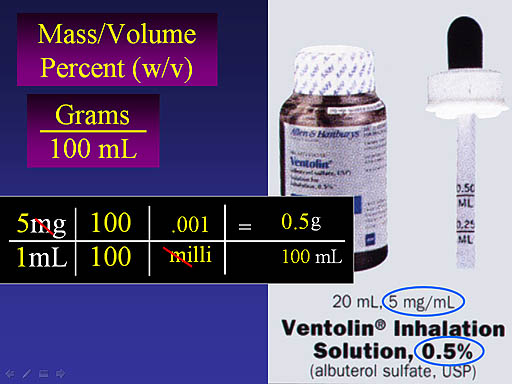
Notice the dimensional analysis starts with 5mg over 1mL. Since we need 100mL, we multiply by 100/100 (which is like multiplying by 1). To get rid of the "m" we multiply by 0.001 over "milli" The "milli" cancels and we end up with 0.5 g over 100mL, which is 0.5% w/v. They don't always show the "w/v" but they should.

Here's an example where they don't indicate "w/v" which is poor practice but commonly done. We will assume the % means % weight to volume.
Let's calculate how much glycerin is in this bottle. At 0.2% that means 0.2g/100mL. By multiplying by the volume of the bottle (15mL), the milliliters (ml) cancels and we get 0.03 grams of glycerin in the bottle. Actually there's very little of anything in the bottle, which makes you wonder why they charge so much.

Here's a company that sells IV solutions. A common one used for dehydration and to give a patient calories is Dextrose 5%. It lists the ingredients as 50 grams of Dextrose monohydrate diluted with water to 1000mL. So that is 50g per 1000mL. Our dimensional analysis starts with that fraction and multiplies by 0.1 over 0.1 in order to get 1000mL down to 100mL. This also turns 50g into 5g. So we end up with 5g per 100mL, which is another way of saying 5% (w/v). Now we know why it's called Dextrose 5%. They also sell Dextrose 10%, which is close to putting Coca-Cola into our veins (as far as sugar concentration is concerned)


So far, we've talked about a solid dissolved in a liquid. Here we have a liquid (alcohol) dissolved in another liquid (water). The concentration in these cases is often volume percent (%v/v). On this bottle it says, "Alcohol 10% by vol." That means 10% of any volume of this champagne is alcohol. So if the whole bottle is 750 milliliters, then 10% of that is alcohol giving us 75 milliliters of pure alcohol.

Other liquors list the alcohol concentration as "proof". 100 prove is actually 50% v/v alcohol. Here the Kahlua is 53 proof which is 26.5% v/v alcohol. So 26.5% of the 750 ml is alcohol.

In chemistry, it's more convenient to
list the concentration in moles per liters. Remember moles is how we count
molecules. With a count we can use a chemical equation to count other
products or reactants in that equation. So that's why we often see chemicals
dissolved in water measured in moles per liter . This is given the name
"molarity or molar" which is abbreviated as a capital M.
In other words, a liter
of the solution on the right that says "1 M
K2CrO4" contains 1 mole
of K2CrO4 (potassium chromate). One liter of the
solution on the left (KOH) contains 0.80 moles of KOH. So this is better
than giving the concentration in grams. If in grams per 100mL, you would
have to look up the molecular weights to calculate moles. This saves that
step.

Here's a 0.1 molar solution of sodium arsenate. That means 1 liter of this solution contains 0.1 moles of sodium arsenate.
To make up 2 liters of this solution you would have to know the molecular weight of sodium arsenate (Na3AsO4), which means consulting the Periodic Table to find the molar mass of sodium (times 3), the molar mass of arsenic, and that of oxygen (times 4). Those add up to 208 grams per mole of Na3AsO4. This solution is only 0.1M, so that means we only need one tenth of a mole for every liter. So we would use 1/10 x 208 grams=20.8 grams for each liter that we want. This is a 5 liter jug, so that means 20.8g/L x 5L = 104 grams. So 104 g of Na3AsO4 will make us 5 liters of 0.1M sodium arsenate solution.

While you have the above calculations fresh in your mind, let's do a few problems of that type.
Here is 0.1M (0.1 moles per liter) of
sodium phosphate.
Problem 1: This bottle has 2 liters
of solution. How many moles of sodium phosphate is in those 2 liters?
Problem 2: If you wanted to make up 1 liter of this solution, how many grams of sodium phosphate (Na3PO4) would you weigh out.


Problem 4:
You are working in a rural clinic where you have to make up your own IV solutions. You are asked to make up a liter of 5% w/v solution of this anhydrous Dextrose. How many grams of this dextrose do you weigh out?

Problem 5:
Pick the best answer.

Problem 6:
We’ve heard that wax or oils repel water. But that isn’t true. Water is so attracted to other water molecules that anything between them is squeezed out of the way. Why is a water molecule attracted to other water molecules? (choose best answer)

Problem 7:
The ingredient states 0.14% wt/vol of
fluoride ion. If you use 1 cc of toothpaste to brush, how many grams of
fluoride ion is present?

Problem 8:
For every pound of this insecticide, how
much of it is diazinon?

Problem 9:
Which of these answers is NOT the concentration of sugar.

Problem 10:
If this bottle is one half liter, how many moles of silver nitrate does it contain? Choose a, b, c, or d.
Extra Credit:
If this bottle is one half liter, how many grams of silver nitrate does it contain?
Extra Credit:
Why is silver nitrate put in a dark bottle instead of a clear bottle?