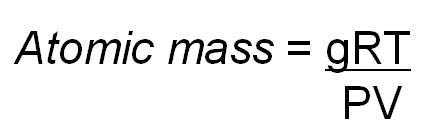The warnings
on all spray cans will say something like this: "Contents under
pressure. Do not puncture, incinerate, or expose to temperature above 120F.
Heat from sunlight, radiators, stoves, hot water, and other heat sources
could cause container to burst."
Problem 3:
Going from 100°F(311K) to 150F(338K) is only
a 8% increase of temperature and also only a 8% increase in pressure if
the container holds only gas. However, some left over paint in the can will cause the pressure to go much higher. Why is that? |
|
Problem
4: 520 gas cylinders containing 300,000 pounds of chlorine gas
was used as a chemical weapon at Ypres, France in 1915. 5,000 soldiers died
and 15,000 injured.
4a) Air weighs 28.8 grams per mole. How many grams per mole does chlorine
gas (Cl2) weigh?
4b) Will chlorine gas stay close to the ground or rise up into the air?
4c) How many grams is 300,000 lbs mentioned above, knowing that 1 lb = 454 grams.
4d) How many moles of Cl2 is 300,000 lbs of Cl2? (use grams from
4c).
|
 |
|
The results of a basketball game was called into question
with allegations of the basketball being filled with a gas other than
air. Your task is to investigate. The ideal gas law PV=nRT may help with
some rearranging. Divide both sides by n gives PV/n=RT, now divide both
sides by PV to get 1/n=RT/PV. Now multiply by both sides by grams to get
g/n=gRT/PV.
"g/n"
is grams per mole and that is what the Periodic Table lists for each element
(atomic mass). So the final formula would read:
Atomic wt=gRT/PV
Calculate gRT/PV and match it to the element that has that
atomic mass.
To calculate you need g,
T, P, & V.
You measured the radius as 12.4 cm. Using formula for sphere V= 4/3
x pi x r3, you calculate its volume as 8,000. mL. The pressure
is 29.4 psi and the temperature is 81°F (27°C,300K) You weigh
the basketball and it weighs 600.65 g. You let out the gas and find the
empty weight as 600.00g.
Problem 6: What is the gas they
put in the basketball? (Hint: turn 29.4 psi to Atm (14.7psi=1atm), convert
8,000mL to liters, use Kelvin, and use 0.0821 for R). |
|