
Oxygen in the air is diatomic (two atoms connected), but chemists don't call it dioxygen, the correct name is oxygen. In the medical field they usually call it "O-two", "medical oxygen", or simply oxygen. Likewise, other gases like fluorine (F2) and chlorine (Cl2) are simply called fluorine and chlorine; it's understood that they travel in pairs.

Sodium chloride is a typical binary ionic compound. "Binary" means there are two elements bonded together. "Ionic" means each are ions (Na+ and Cl-). Since opposite charges attract, this causes them to bond (combine) to make a compound. When talking to patients with high blood pressure, medical staff will say, "Reduce your intake of salt." This use of "salt" means sodium chloride (NaCl).
Salt is a general term for any metal combined with any non-metal. However, in everyday language, this word usually means sodium chloride.
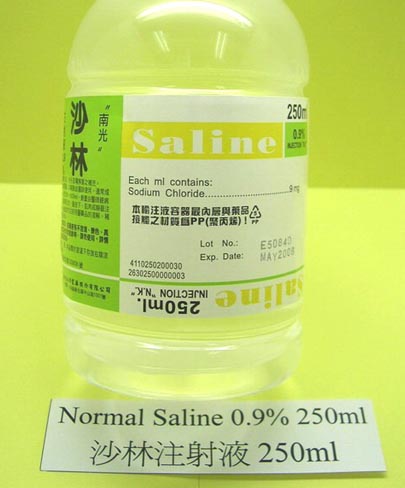
Saline is a term the medical profession uses for a sodium chloride solution. "Normal Saline" means a sodium chloride solution that is 0.9% w/v [weight/volume]. 0.9% w/v means 0.9 grams per 100 mL of solution. You can make it by putting 9 grams of sodium chloride in water and then bring up the volume to 1 liter.
"Normal" means that the salt level is close to the "salt" or "ion" levels in blood. That's why normal saline is given to dehydrated patients who can't take liquids orally. It is administered by IV (intravenous) through injection or use of a IV bag.
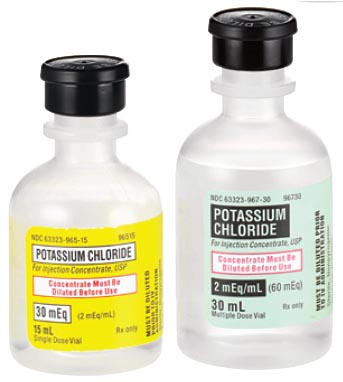
Potassium is needed for the proper functioning of muscles and nerves. If the body doesn't have enough potassium, the condition is called "hypokalemia". "Hypo" means under. "Kal" is from "Kalium" the Latin name for potassium (hence K as symbol). "emia" means blood. So hypokalemia means low levels of potassium in the blood. Potassium chloride is another binary ionic compound. Notice binary compounds end with "ide". The positive ion (potassium, K+) is always written first.

Titanium (IV) oxide is another binary ionic compound. It's used as a sunblock for people sensitive to the sun's UV light or are in high risk environments (mountains, snow, water)
Notice unlike sodium or potassium chloride, this name uses a Roman numeral between metal and non-metal. Sodium and potassium ions are always a plus one charge, so there's no need to indicate charge. Titanium however, can have a plus 2, plus 3, or a plus 4 charge, so there needs to be a way to indicate which one. The Roman numeral IV means this titanium has a plus 4 charge. To balance that charge, you would need two oxygen ions (oxide ions) because each oxygen ion is a negative 2 charge. So the formula is TiO2. This product names this compound as titanium dioxide, which is logical since there are two oxygen atoms; however, the organization that has the task to standardize chemical names says using the Roman Numerals will reduce misunderstandings. So the proper name is titanium (IV) oxide, but you are apt to see and hear it as titanium dioxide.

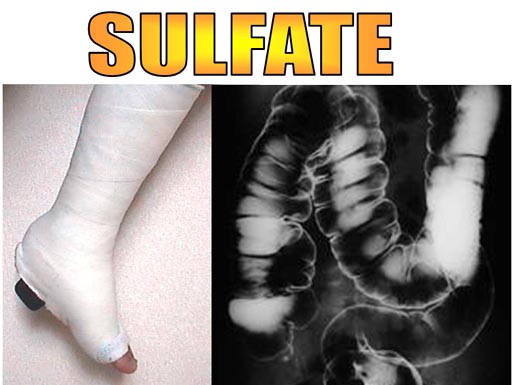
In the medical profession calcium sulfate and barium sulfate are often encounter.
Casts are made from calcium sulfate hemihydrate. (CaSO4 * 0.5H2O). "Hemi" means one half and "hydrate" means water. In other words, one water sits between two CaSO4 molecules.
Barium sulfate is taken orally (like a bad tasting milkshake)
and is used to absorb xrays. This allows the outline of the digestive
tract to be visible in xrays.

Sulfite: Sulfate has four oxygen atoms. If there are only 3 oxygen atoms, then the name changes to "sulfite". The formula is "SO32-". It still has a negative (minus) 2 charge. This ion is important in health care because 1 out 100 people are sensitive to sulfite causing sometimes severe allergic reactions. Some people have died. The normal allergic reaction is breathing difficulties. Sulfite is used as a preservative in wines and many foods (like trail mix).
Sulfites are used to sterilize fermentation equipment and food containers because of its antimicrobial properties. Generally meat, cereals and dairy products are not treated with it as it destroys the thiamine (vitamin B1) content.

Most products don't list sulfite as an ingredient. They
will call it sulfur dioxide (or British spelling sulphur dioxide). Sulfur
dioxide is a gas. If you opened the bag, the sulfur dioxide would escape
and no longer be a preservative. However, there's some moisture in trail
mix or even "dried" fruit. Plus, there's water in wine. So the
sulfur dioxide they list has actually reacted with water. Here's the reaction:
SO2 + H2O -> H2SO3
The product is called sulfurous acid. Now you see why they don't want
to label it as sulfurous acid, which is very close to sounding like sulfuric
acid.
In water, the sulfurous acid breaks up into ions with one being sulfite:
H2SO3 -> 2H+ + SO32-
Now you can see the sulfite ion that some people are allergic to.

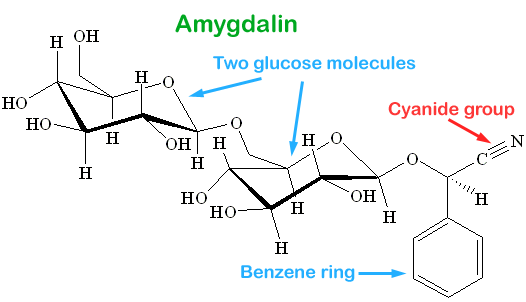

The type of cyanide used in gas chambers was hydrogen cyanide. It is a gas. As it got into the lungs and contacted water, it would break up into hydrogen ions and cyanide ions.
The other poisonous forms of cyanide are the sodium and potassium salts of cyanide. NaCN and KCN. These were used in suicide pills that spies carried with them.


ACETATE:
Vinegar has been used for centuries to combat microbes in foods. That's
the basis of pickled foods. Vinegar in stores is a 5%
w/v (5 g/100mL) solution of acetic acid. In water some of the acetic acid
loses a hydrogen ion (acid) that gets grabbed by water.
HC2H3O2+
H2O -> C2H3O2-
+ H3O+
The negative ion of acetic acid is called "acetate". The water that has grabbed the H+ ion is called the hydronium ion.
Also in the picture is a bottle of zinc acetate, which is a cold remedy. The bag contains sodium acetate. See next row for more information.

NaC2H3O2
Sodium acetate is used in instant heat packs. The way it works is very interesting chemistry. When heated, sodium acetate melts, but when it cools back down to the temperature when it was solid, it doesn't go back to being a solid. It stays a liquid.
A single crystal of sodium acetate or a disturbance from the "activator button" can cause the liquid sodium acetate to turn to a solid. At that point heat is released. The reason is that molecules in a liquid are moving around, but molecules in a solid are not moving around. Motion is energy, more specifically kinetic energy. When these molecules come to an abrupt halt as they go from liquid to solid, that kinetic energy has to go somewhere. That's the heat that is given off.
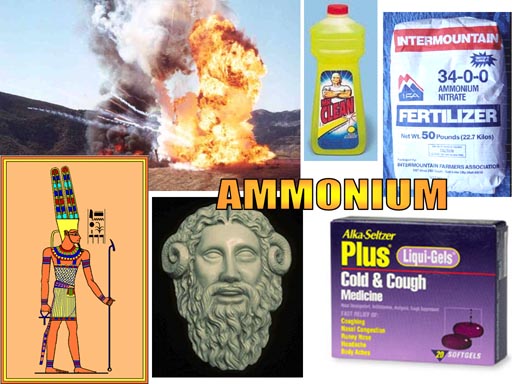
Nearly all polyatomic ions you encounter are negatively charged. Ammonium is one that is positively charge. Ammonium gets its name from the Egyptian Ram God, Ammon. A salt with special properties was discovered near the Temple of Ammon. It was called Sal (salt) Ammoniac (NH4Cl). When heated, it decomposes into ammonia and hydrogen chloride. NH4Cl -> NH3 + HCl.
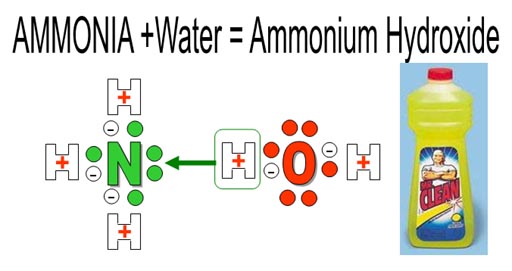
Other images: Ammonium chloride is used in cough medicine. Ammonium nitrate is used as a fertilizer and as an explosive. Ammonium hydroxide (NH4OH) is used in cleaners.
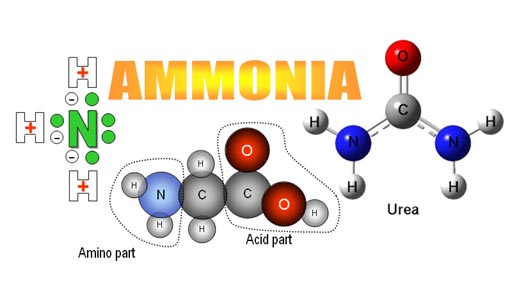
Ammonia is created in the body from digestion of proteins and amino acids (middle image: notice the amino part of this amino acid). If there’s an excess of nitrogen, the body converts it to urea, which is less toxic than ammonia. Urea is expelled in the urine.
Some babies are born without the enzymes to convert
ammonia to urea, so they develop hyperammonemia, which is fatal or
will cause brain damage if not treated. A case story about this disease
is here: http://www.glennon.org/archives/

Bicarbonate (HCO3-):
In chemistry the new preferred name is hydrogen carbonate; however, you will probably see the name, bicarbonate, persist in the medical field for a long time.
Sodium bicarbonate (NaHCO3) is used in emergency situations like heart attacks, kidney failures, and lung problems to correct a pH imbalance from too much acid in the blood. Here's the chemical equation of neutralizing acid (H+):
H+ + HCO3- -> H2CO3

CO2 + H2O -> H2CO3 -> H+ + HCO3-
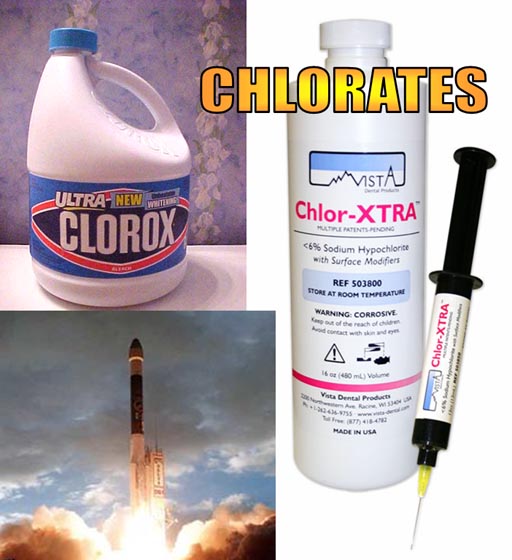
Chlorates: Chlorates are a group of polyatomic ions that are have one chlorine atom and one to four oxygen atoms. (ClO-, ClO2-, ClO3-, & ClO4-). The names in same order are hypochlorite, chlorite, chlorate, and perchlorate.
In the medical field, sterilization is always critical. One chlorate, sodium hypochlorite (NaClO), is the main ingredient in Clorox and is commonly used as an disinfectant. A syringe with NaClO solution is used in dentistry to clean and disinfect root canals.
Chlorate, and perchlorate are also used as a source of oxygen in fireworks and in rocket fuel. Perchlorates from dumped rocket fuel have contaminated the water supply in several states. This is a health issue currently being examined.

Nitrate (NO3-) & Nitrite (NO2-): These are produced by bacteria feeding off of human and animal waste. Nitrate can also come for fertilizers such as ammonium nitrate. High nitrate levels in drinking water can harm infants. Nitrate is converted into nitrite by bacteria that survives in infants’ stomachs. The nitrite ion enters the blood and converts the hemoglobin in red blood cells to methemoglobin The "met" is from "meta" meaning "changed." This "changed" hemoglobin has less ability to carry oxygen, causing a condition known as methemoglobinemia, also called "blue baby syndrome."
a) NaCl
b) TiO2
c) NaNO3
d) KNO3
e) NaCN
f) NaClO
g) KClO4
h) Na2CO3
Problem 1:
Give the written name of these binary & polyatomic ionic compounds.
a) Potassium chloride
b) Titanium (II) oxide
c) Calcium carbonate
d) Sodium bicarbonate
e) Iron (II) sulfate
f) Calcium sulfate
g) Ammonium chloride
Write the formula for these binary & polyatomic ionic compounds.
CO2
SO2
HCN
Write the written name for these non-metal compounds.
Carbonic acid
Sulfurous acid
Acetic acid
Write the formula for these acids.

At one time it was legal to spray salad bar items with a sulfite solution. This helped preserve the vegetables and fruit and kept them from turning brown. However, after a few patrons died and others were hospitalized, restaurants are banned from using sulfites or sulfur dioxide on salad bar items. What is the health problem with sulfite?

Problem 6:
Ammonium carbonate is sold here as a leavening agent for pastries. Leavening
causes the dough to rise because it produces gases. Notice all the warnings.
Ammonium carbonate is also used for smelling salts. The formula is (NH4)2CO3.
What dangerous gas is being released when this compound is used in baking?