
Reading:
Instructions will be given for both 11th
and 12th editions of the book.
11th: pages 83-96
12th: pages 84-98
It's a short chapter, so read the whole chapter. My tutorial will reinforce a lot of what you read; however, there are some extra information in the textbook.


Table 5.1: 11th-page
86, 12th-page 87
The textbook shows a
table of the percent composition of various compounds. Unfortunately,
it made the same mistake as in a previous chapter of not saying what kind
of percent. Remember a compound is made up of two or more elements. Se
each element makes up only a fraction of the whole compound. For example,
the upper right molecule is water (H2O). If the percent is
by number then hydrogen is 67% because it is two out of three atoms. If
the percent is by weight, then hydrogen is 11% because their weight is
only 11% of the whole molecule's weight. The table shown in the textbook
is percent composition by weight. The reason percent by weight is used
is that the early methods for analyzing compounds was to break the individual
elements away from the compound using heat or chemicals. Then weighing
what was left or the new compounds formed. This was called an assay. A
common assay was to determine the percent of silver or gold in an ore.

Problem 1:
The "Isotope Detectives"
is an interesting story for identifying the origin of cocaine and emeralds
using isotope ratios. For emeralds they talk about analyzing the oxygen
in the emeralds. Remember, in the building block tutorial on inorganic
compounds, oxygen was the most common building block.
(1a) What is the formula for an emerald? (You can use Wikipedia).
(1b) What are the 3 most stable isotopes of oxygen and what percent is each one?
(hint: use Wikipedia, look at bottom of right table)
Problem 2a:
11th edition: page 98, question 1.
12th edition: page 99, question 1.
What are the atomic numbers of (a) copper,
(b) nitrogen, (c) phosphorus, (d) radium, and (e) zinc.
Followup question: Problem 2b: How many protons
are in each of the elements listed?
Problem 3:
11th edition: page 98, question 4.
12th edition: page 99, question 4.
Distinguish between an atom and an ion.
Problem 4:
11th edition: page 98, question 6.
12th edition: page 99, question 6.
In what ways are isotopes alike? In what ways are isotopes different?

Problem 5:
11th edition: page 99, question 19.
12th edition: page 100, question 11.
(5a) What special names are given to the isotopes of hydrogen?
Followup question (5b) : The isotope of hydrogen that has two neutrons is radioactive; however, you can buy it. Where?

Problem 6:
Not in textbooks.
Visit the Wikipedia page on Radiopharmacology.
wikipedia.org/Radiopharmaceutical
They list about 25 radioactive elements that are used in medicine. Notice
that radioactive elements are written in two formats. For example, the
radioactive form of sodium is called sodium-22 or 22Na. Radioactive
elements are used in a few ways. One use is for getting images from within
the body. This is possible because radioactive elements give off either
gamma rays (high energy x-rays), high speed electrons (called beta particles),
helium nuclei (called alpha particles), or positrons (called positive
electrons or anti-electrons). Because something is emitted, these radioactive
elements can be detected using different kind of cameras or sensors. So
they are used to make an image of what's going on in the body.
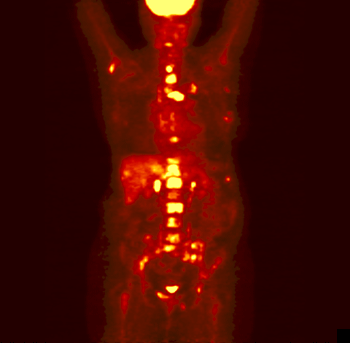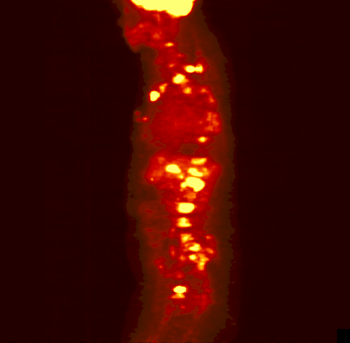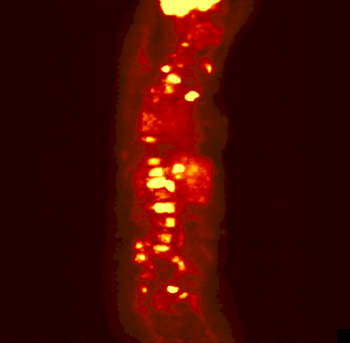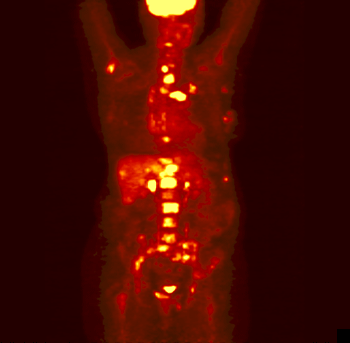
The rotating image is a person who has
been injected with a sugar or other compound built with some atoms that
are radioactive. These particular atoms emitted positrons. As the positrons
come in contact with electrons in the body, the positrons and electrons
annihilate each other forming pairs of gamma rays. Those get detected
and eventually made into this image.
(6a) Which of the elements listed are positron
emitters? (list name and isotope number).
(6b) Carbon-11 is a positron emitter. What
parts of the body is carbon-11 used to generate an image?
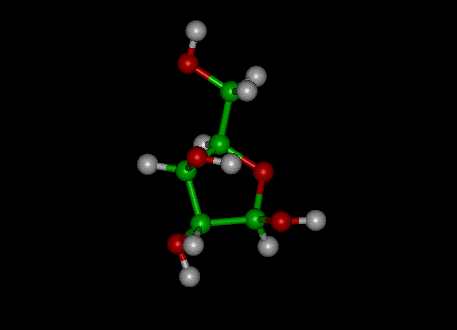
Problem 7: Still based on Radiopharmacology article in Wikipedia.
To the left a sugar called d-xylose. One
of the carbons (green spheres) is replaced with radioactive carbon-14.
D-xylose is food for bacteria in the intestines.
(7a) What are they investigating using carbon-14
in d-xylose?
(7b) How is this sugar administered?
(7c) It says that the test is done "in-vitro".
What does that mean?
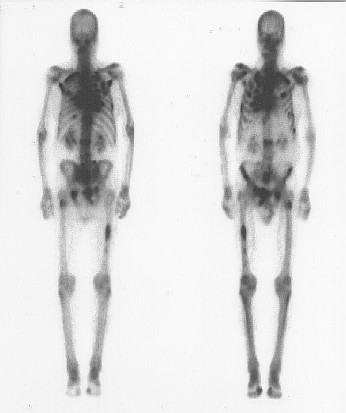
Problem 8: Still based on Radiopharmacology article in Wikipedia.
We can't wrap up these questions without attention paid to one isotope: Technetium-99m. Here is a quote from Wikipedia: "Technetium-99m is used in 20 million diagnostic nuclear medical procedures every year. Approximately 85 percent of diagnostic imaging procedures in nuclear medicine use this isotope." This images from this isotope looks like x-rays because that is what this isotope produces. Unlike an x-ray image, which is a shadow of the body as x-ray light shines through it. These images are from xrays that come from within the body. The Technetium-99m is attached to various chemicals or even red blood cells. Once put into the body, it will collect at certain sites in the body. So these "xrays" tells us about the chemistry and workings going on in the body. For example, red blood cells can be taken from the body, attached to technetium-99m, and then put back into the body. These red blood cells will be emitting their own xrays which can be photographed or watched on certain screens. (Problem 8) What 3 types of investigations are done using red blood cells doped with technetium-99m? (also explain what they mean. Hint: the second one listed is blue, meaning if you click on it, it will go to another article about it).