

<-11th Edition 12th Edition->

13th Edition
Instructions are given for the 11th, 12th, and 13th Editions.
You'll find that textbooks will mention names or formulas for substances in their questions but not say what that substance is. You'll find that I want you to look up what they are talking about. Being at the computer, it's easy to do that.

Be sure to go through my online tutorial
of "Classifying: Calming the Chaos" first.
I find it interesting that the 11th edition had the same idea I had about chaos. Here is a quote from that textbook. It's the first two sentences of Chapter 3.
"Throughout our lives, we seek to bring order into the chao that surrounds us. To do this we classify things according to similarities."

Chapter 3:
Elements and Compounds:
11th Edition, p44-60
12th Edition, , p47-60
13th Edition, , p43-55
Read the whole chapter. Much is covered in my online tutorial, but there are some addition information here.

Percent distribution:
11th Edition, section 3.5, pg 49-51:
12th Edition, section 3.2, pg. 49-50
13th Edition, section 3.2, pg. 45-46
In this section the textbook lists the percent distribution of elements
in the universe, Earth's crust, and human body. This is interesting, but
they left out a critical piece of information in the 11th and 12th editions. For example, they said
hydrogen makes up 10% of the human body. 10% means 10 out of a 100; unfortunately,
they didn't say whether this was by weight or by number. In other words,
they didn't say if this was 10 atoms of hydrogen out of 100 atoms of the
body (by number) or was this 10 grams of hydrogen for every 100 grams
(by weight) There's a big difference.
To find out let's visit webelements.com. If you follow this
link you will see that they give two numbers for abundance: ppb by
weight and ppb by atoms
(number of atoms). "ppb"
means parts per
billion. Remember "%" means parts
per hundred.
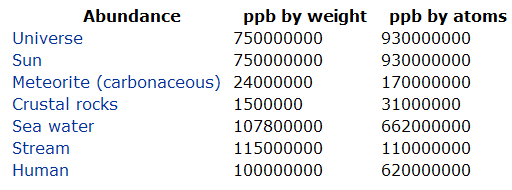
This is the chart from the webpage. Notice for humans
hydrogen is given as 100000000 ppb by weight. That's a fraction of 100
million over 1 billion. That reduces to 1/10, but for percent we need
over 100.
100,000,000
= 1
= 10 = 10%
1,000,000,000 10 100
The textbook said 10% so now we know they meant
10% by weight meaning 10% of the weight of the body is hydrogen. Also,
all of their other listings of percent is meant to be by weight. Shame
on them for not being more specific. At least in the 13th Edition they corrected that oversight.

Note we are sometimes interested in what fraction of the
total number of atoms is hydrogen. Webelements gives that fraction as
620000000 ppb. That's a fraction of 620,000,000 over 1 billion. That reduces
to 62/100, which is 62%
620,000,000
= 62
= 62%
1,000,000,000 100
62% is very different from 10%,
so it's extremely important that when % is given in chemistry, one clarifies
what are the units. Remember dimensional analysis depends on the units.
Problem 1: What percent
of all atoms in the universe are hydrogen atoms?
Problem 2: Chemical
formulas :
11th Edition, Question 37, page 62
12th Edition: Question 7, page 62
13th Edition: Not in this edition, but problem is shown below.
Explain the meaning of each symbol and number in these formulas.
(a) H2O
(b) Na2SO4
(c) HC2H3O2
Problem 3: Regarding (b), what is the name for
the compound Na2SO4? Hint: use search engine
and type in Na2SO4.
Regarding (c), when you see "H" written twice,
that usually means that the compound is an acid. An acid has one or
more hydrogen atoms that get released into water as H+. That's what
makes it an acid. Those hydrogens that get released are usually written
separate from the hydrogens that don't come off.
Problem 4: What acid is HC2H3O2? Hint: use a search engine and type in HC2H3O2 and look for a name of an acid being shown.
Problem 5: Total
number of atoms in compound:
11th Edition, Question 39, page 62
12th Edition: Question 9, page 62
13th Edition: Not in this edition, but problem is shown below.
How many (total) atoms are represented in each formula? (just give
total atoms for each formula)
a) KF
b) CaCO3
c) K2Cr2O7
d) NaC2H3O3
e) (NH4)2C2O4
Problem 6: Regarding (b) CaCO3. What
is the chemical name for CaCO3? (remember hint before). Also,
check out Wikipedia's article on its name and the first paragraph mentions
a medicinal use for CaCO3. What is it medicinal use?
Problem 7: Regarding (d) NaC2H3O2. 7a) What
is the chemical name for NaC2H3O2? (a Google search will find it, but I discovered that typing nac2h3o2 into Wikipedia is much easier). 7b) Also,
in the Wikipedia's article report one way in how it is made (see Preparation heading).
Problem 8: Atoms
of Oxygen:
11th Edition, Question 41, page 62
12th Edition: Question 11, page 62
13th Edition: Not in this edition, but problem is shown below.
How many atoms of oxygen are represented in each formula?
a) H2O
b) CuSO4
c) H2O2
d) Fe(OH)2
e) Al(ClO3)3
Problem 9: (a) H2O is something you know,
but what is the chemical name for (c) H2O2?
Problem 10: When
you find the name, look it up in Wikipedia. Read the first paragraph and
then in the Contents listing, click on Therapeutic use. Report 3 uses
for H2O2.

You should recall in the earlier tutorial titled "Symbols vs. Real" I wanted you to always be aware of what are symbols and what are the real things. A textbook is full of symbols in the form of words, numerals, element symbols, formulas, and many other symbols. The only real elements the textbook can show are carbon, oxygen, and hydrogen with some silicon and aluminum. That's the elements in paper. The compounds in paper are cellulose (long chains of sugar) and clay or chalk. Chapter 3 introduces the symbols of elements and compounds.
Problem 11: What elements are represented in these "words"?
(a) COKEs (b) CoKEs
(c) TiDy
(d) KrYPTiC (e) SnOB
Since words and chemistry both share the same set of symbols, which is the English alphabet, there's a way to write words and at the same time be writing element symbols. This problem was to make you aware of the importance of upper and lower case. In chemistry, that can easily change which element you are representing.
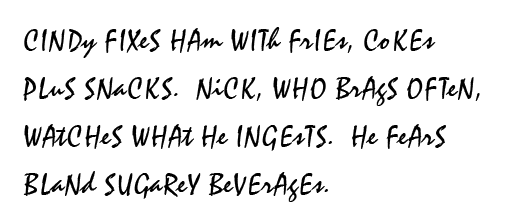
In the sentence shown, it seems that we are reading about Cindy and Nick; however, it's really just symbols for elements. I can see that spies could use this technique to communicate formulas or elements by disguising them as sentences. For example, the formula for the strongest permanent magnet is NdFeB (Neodymium, iron, & boron).
Problem 12: Which words
in these sentences have the symbols for these three elements (hint: there
are 2 words)?
Problem 13: What elements are listed in the series of element symbols that look like the word "beverages"?