
The Titanic is good reminder of these topics. It was the cold temperature that made the steel more brittle when it struck the iceberg. The cold temperature also killed most of the people who ended up in the ocean. The mass of the ship was so much that they couldn't steer it away from the iceberg. The volume of water that leaked in canceled out the buoyancy of the air. The density of iron guaranteed that it would sink.


Temperature:
How do you invent a way to measure temperature?
Most objects expand when heated, so you might relate the amount of expansion to the temperature. A metal bar will expand a little when heated but it takes a lot of heat to see any expansion.

Water Thermometer:
What about the expansion of a liquid?
Galileo made a rudimentary thermometer based on water expansion in 1593.
When the water got hotter, it would expand and travel
a small ways up the tube. But this thermometer was not very accurate because
the distance the water rose wasn't very much.
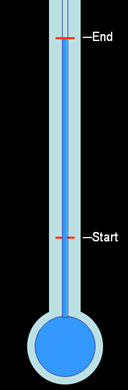
Narrow neck thermometer:
To exaggerate the distance the water would rise, the water was allowed to expand in a narrow channel. This meant the expansion was more noticeable because the height of the water would change much more for the same amount of expansion due to heat.
The only draw back is that water has a narrow range of temperatures that it could measure. If too cold, it would freeze. If too hot, it would boil.
If alcohol was added to the water, that would extend the temperature range some. If red dye is added, then you could see it better. This is a common thermometer used today. However, it still had didn't have enough temperature range for, let's say, an oven.
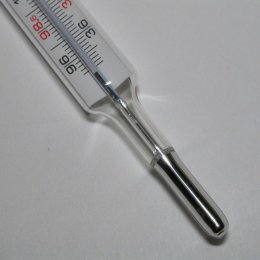
Mercury Thermometer:
Mercury was a better choice. It had a high boiling point and a low freezing point.
So for a couple of centuries it was the thermometer of choice until we learned mercury was quite toxic. Now these are replaced with the alcohol type unless higher temperatures have to be measured.

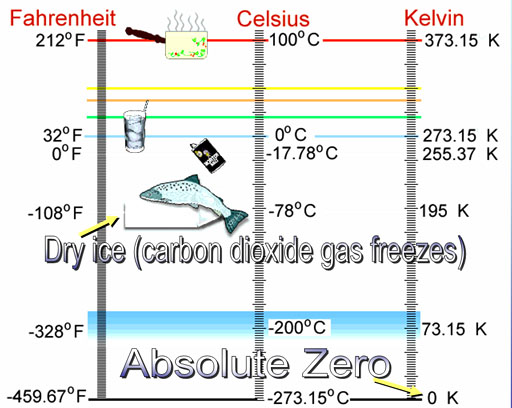
Daniel Gabriel Fahrenheit (1686-1736) from Amsterdam placed three marks on his mercury thermometer. The lowest temperature that he could create in the lab was a 50:50 mixture of ice (snow) and salt (ammonium chloride). He assigned this as zero degrees. In making ice cream, we use ice and salt (sodium chloride) to get it cold enough to freeze ice cream.
Another mark was the freezing point of water. The highest mark was the boiling point of water. He liked the number 180 because it was half a circle in degrees. So he divided up the distance from freezing to boiling by 180 steps (degrees). At that size of steps, freezing was 32 degrees above zero on his thermometer. 32 plus his favorite number of 180 puts boiling at 212. This scale is named after him.
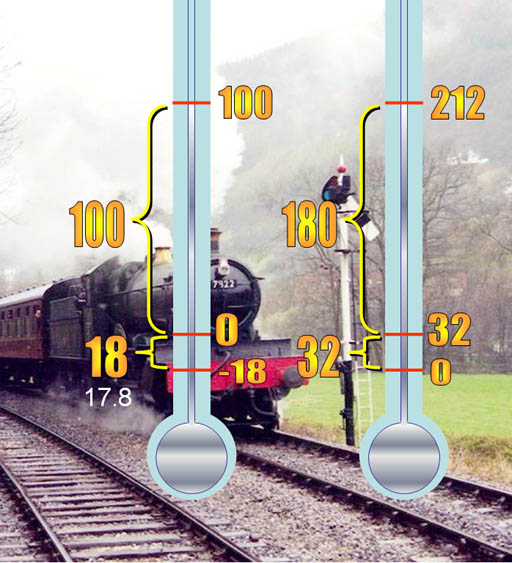
Celsius: Swedish astronomer Anders Celsius (1701-1744) thought the zero mark on the Fahrenheit thermometer was hard to reproduce because of variations in salt and ice. He decided pure water was the easier to get and use to calibrate thermometers. He set freezing to zero and boiling to 100.
The Celsius degrees are almost twice as large as Fahrenheit degrees because Anders Celsius broke the freezing to boiling range into 100 steps instead of 180 steps.This shows how we can convert from one to the other. If starting with Celsius, we need to multiply by 180/100 (9/5) because there's 180 Fahrenheit degrees for every 100 Celsius degrees. Then we add 32 because Fahrenheit's zero is 32 degrees below Celsius' zero.
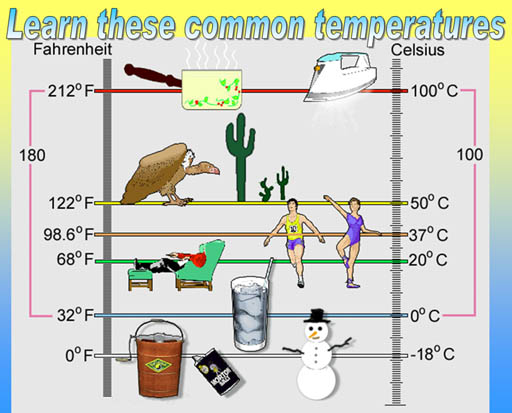
Learn these common temperatures:
The top temperature is the boiling point of water. 50°C
is the hottest day on record in Phoenix, Arizona. 98.6°F (37°C)
is the temperature of the body. 68°F (20°C) is considered room
temperature. Most glassware for labs are calibrated to be most accurate
at 20°C. 32°F (0°C) is where water coexists as liquid water
and ice (freezing point). 0°F (-18°C) is temperature of a mixture
of ice and salt.
Even though we these scales had zero degrees, that didn't mean temperature
could not go colder.

How cold can we go?
Temperature is the average energy of the movement of atoms
or molecules. The slower they move, the cooler the temperature.
So if movement defines temperature, when movement stops, that’s the
coldest temperature can get. That temperature is called absolute zero
because that is absolutely as cold as you can go.
Absolute
Zero:
The Kelvin scale, named after Lord Kelvin who proposed
it, sets zero at absolute zero, the temperature where all atoms stop moving,
spinning, or vibrating. Electrons & nuclei, however, continue to move.
Notice these low temperature markers. Dry ice is at -108°F (-78°C/195K).
Other gases in the air (oxygen, nitrogen) start turning to liquid around
300°F below zero. Everything is solid at Absolute Zero.

A lot of interesting things happen when items are at very cold temperatures. A common occurrence in superconductivity, which means electrical current can flow without any resistance. For example, when a magnet drops onto this supercooled metal that is superconducting, the falling magnet generates electric currents in the metal that creates a virtual mirror magnet. This virtual magnet repels the real magnet causing the real magnet to levitate. This will go on forever until the metals warms up and loses its superconductivity.

MASS:
An object has mass if...
1) it takes up space.
2) it is affected by gravity.
3) resists being moved. (sped up or slowed down)

WEIGHT
vs. MASS:
This big dirigible weighs nothing because it is hovering. However, it still has a lot of mass. Even though we use these terms somewhat interchangeably, they aren't the same. Weight is a force that mass exerts under the influence of gravity. Here its weight is canceled by the buoyancy (upward push) of the weight of the atmosphere.

Weight depends on gravity:
Most of the devices we use to measure mass are actually measuring the force that gravity has on the mass. That force is called weight and is related to the mass. However, none of these items could measure the mass if they were in the weightlessness of space.

On level ground, gravity has no effect on the difficulty of pushing a heavy car. Just the car’s mass is felt.
If the girls push with the same strength, one can measure the distance the two cars moved after, let's say, 5 seconds. With this informaiton we can calculate mass.

Why is "lb." used for pound?
The Latin word libra describes
a Roman unit of weight similar to the weight of a pound, and they used
the abbreviation "lb " for that
unit of weight and the sign £ (a crossed-out
L) for the currency derived from this. The word "pound" itself
comes from the Latin pendere, to weigh.
In Greek mythology, the Libra constellation
depicts the scales held by Astraea ("star-maiden" ) (the Virgin),
the goddess of justice
Which is heavier?
Which is heavier a pound of feathers or a pound of lead? (roll cursor over image for lead).
The answer is neither, because they both weigh a pound.
This riddle reveals a confusion that some people have regarding weight versus density. Density involves weight but that alone is not enough.
Which is heavier if same volume?
Which is heavier a cubic centimeter of feathers or a cubic centimeter of lead? (roll cursor over image for lead).
When we specify a certain volume, we are less fooled. We realize that for the same volume, lead will be a lot heavier. In other words, lead is more dense than feathers.
Density considers the mass (or weight) AND the
volume that mass occupies.
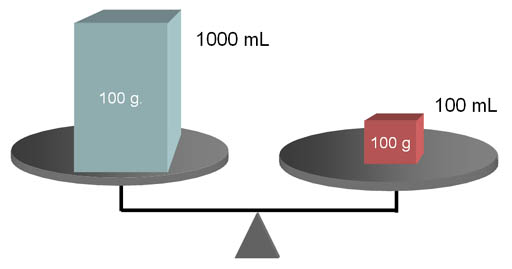
DENSITY COMPARISON:
Objects may have the same weight (notice they are balanced),
but not have the same density. You can look at these and tell that the
smaller red box has a higher density than
the blue box. It has the same mass but packed into a smaller volume.

DENSITY:
A convenient way to compare densities is to find the grams in one milliliter (grams for each milliliter). Dividing by the 1,000 or the 100 changes both of these to have a volume of one mL.
1.00g/mL means 1.00grams per 1 milliliter.

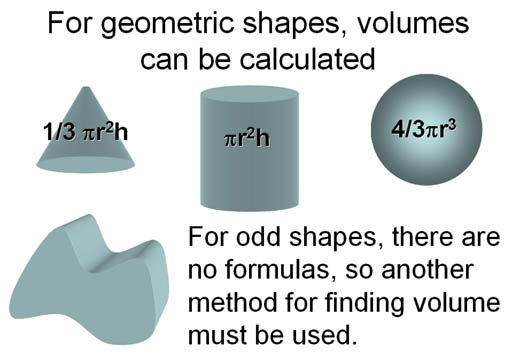
Finding Volume:
To find the density of an object we need mass and volume. Volume can be calculated from the dimensions if it's a geometric shape. However, odd, irregular shapes have no formula for volume, so we need another method.

ARCHIMEDES:
The person who solved the dilemma of finding the volume of an irregular shaped object was Archimedes (c. 287 BC – c. 212 BC.)

LIFT THE EARTH:
So vast was his knowledge of leverage that he once said, "Give me a place to stand on, and I will move the Earth!".

Archimedes Honored:
On this Greek stamp honoring Archimedes, notice the submerged
object attached to a balance. This technique can measure volume and mass,
and therefore density.

A Famous Story about Archimedes:
King Heiro suspected the royal goldsmith had created an inferior gold wreath crown by mixing gold with silver. He ordered Archimedes to find a way to prove the wreath was or was not pure gold.

Knew Mass but not Volume:
Archimedes knew the density of pure gold and the density of pure silver. If the density of the crown was 1.3 lbs per fluid oz, he would know it’s pure gold, but if it’s less than that, then it was mixed with the less dense silver.
He could weigh the crown but could not find its density because he couldn’t measure its volume because of its irregular shape.
He didn’t dare melt it down. He struggled with this problem for a long time until he came up with a plan while getting into a bath tub.

Eureka!:
As he got into the tub, he saw the water rise. He suddenly realized the volume of his body was displacing the water, causing the water level to rise by the same volume of his body. He yelled “Eureka!” meaning “I found it!” (he had found the answer to testing the crown). He ran down the street naked yelling “Eureka!”
Two thousand years later, prospectors for gold would yell, “Eureka!” when they found gold, not knowing that the word is Greek and made famous by Archimedes.
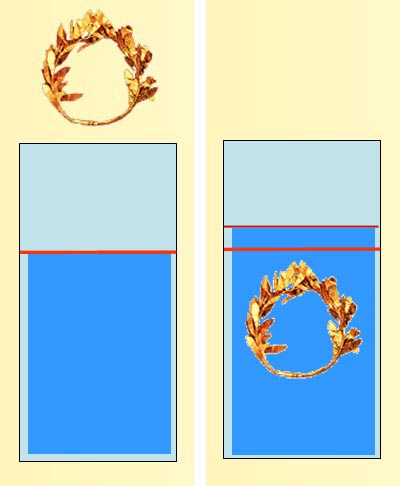
How to measure volume of irregular object:
Before lowering the crown into the water, the level of the water is marked.
The crown is lowered into the water and the water level will rise.
The new level is marked and the volume difference of the water is the volume of the crown. So if the weight was 3.3 lbs and the volume increase was 3.3 fluid ounces, what is the density?
3.3 lbs = 1
lb.
3.3 fl.oz fl.oz.
Is it pure gold?
No, because pure gold is 1.3 lb/oz.
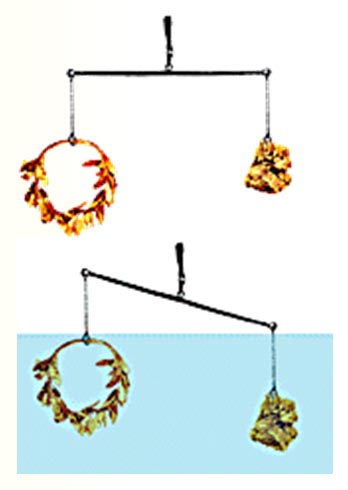
Drum roll please:
A more sensitive and more dramatic approach that requires no calculations is to balance the wreath with equal weight of pure gold. If the wreath is pure gold, both of their volumes should also be equal.
When dipped in water, they should displace the same amount of water and be lighter by that weight of water (equal buoyancy) and stay balanced. However, if the wreath has more volume (because of the silver), it will displace more water, making it lighter than the gold on the other side.
This is what Archimedes discovered. The goldsmith did
cheat King Heiro, and the angry King had the goldsmith executed.

Density is a very useful measurement in chemistry. It is a quick way to help identify a substance.
Example: This university has a program where students build racing cars. Below they show axles can be ordered as titanium or aluminum. Let’s say they got a shipment of axles claiming to be the expensive titanium, but they suspected the parts were made from cheaper aluminum. How could they confirm or disprove the composition as titanium?
Titanium has a density of 4.5 grams per ml Aluminum has density of 2.55 grams per ml
So all they needed to do was find the axles' density.

Finding density takes only three easy steps.
(1) Weigh the object. Grams is preferred.
(2) Find volume by submersion in water. Be sure to note volume before and after the object is submerged. Measure volume as milliliters.
(3) Calculate density by dividing weight (mass) by the volume.
In the problem above, if the density is close to 4.5g/ml, then the axle parts are titanium. If density is close to 2.55g/ml, then the metal was aluminum, and you want your money back.

