|
Copy the questions below (highlight the
text, then press the CTRL key plus "c", or use the Edit menu
at top and choose "Copy")
In
your email program start composing an email and paste the questions to
your email (use CTRL-V to paste or use Edit menu and choose "Paste").
I don't need the images. You can then answer them in your email and send
it to me at chm130@chemistryland.com.
|
| Question 1: Even though extinct now,
the saber tooth tiger probably gave early man much to fear and was a fierce
competitor for food. In what ways do you think the saber tooth tiger was
superior to the humans of that time (last ice age) |
 |
|
Question 2: The image on the right is a boulder
of obsidian. Obsidian was the mineral of choice by prehistoric hunters
for making knives and arrowheads. It is still used today by some surgeons
where the sharpest blade is needed. One company which sells surgical tools
also carries the obsidian scalpel. Visit this Web page and find out the
price for an obsidian scalpel with a 12mm (1/2 inch) blade.
http://www.finescience.com/...obsidian-scalpels.htm
|
 |
|
Question 3: Another mineral used for making stone
tools was flint. Flint is also glass-like. The arrowheads to the right
are made of flint. They are sharper and harder than steel. Flint is so
hard that when it strikes a piece of steel, the flint scratches the steel
and particles of steel fly off white hot as they burn in the air. For
this reason flint was used in the old "flintlock" guns to ignite
the gunpowder. Now it is used in cigarette lighters and in as a foolproof
way to start a fire in wet environments. Do a search of the Web for "flint
firestarters" What did you learn about them?

|
 |
| Question 4: Since "flint"
is a common mineral used for stone weapons and "knapping" is a
German word meaning to "break a piece off", flintknapping is the
art of breaking pieces off of flint (or other suitable minerals) for the
purpose of making stone tools or weapons. Search the Web for flintknapping
and find a person who is a flintknapper and which state are they from. |
 |
| Question 5: Stone arrowheads apparently
were sharp and hard. However, their real effectiveness came from being attached
to an arrow shaft, which could hit something at a good distance. Even today,
many hunters prefer the bow and arrow because of its stealth and lethal
power. Search the Web and try to find the world distance record for
shooting an arrow. |
 |
|
Question 6: Scenario: You are walking along a
dry river bed and come across a dog that has gotten tangled up in some
rope. The rope is too knotted and stiff for you to untie it. You need
a knife to cut the rope but don't have one. What could you do?
|
| Question 7: If this woman was to leave
the grain outside for a few days, what might happen to it? (other than someone
stealing it) |
 |
|
Question 8: Earthenware is clay fired at
a low kiln temperature around 1400-2000°F . Earthenware is not very
strong and is porous.
Stoneware - harder than earthenware, stoneware is fired at about
2200-2400°F. Stoneware is strong and can hold water, though is not
completely water proof unless glazed.
Porcelain - a special type of clay either
white or grey, to which kaolin (a white firing stiff clay) and white China
stone (finely decayed granite) is added. When fired at temperatures of
2300°F and over (up to 2550°F was achieved by the Chinese), the
body vitrifies, i.e. it becomes glass-like and completely impermeable.
Let's say you built a kiln that used aluminum, iron, copper, and chromium
parts. Which of those metals would melt if the oven was taken up to 2400°F
(1315°C)
(hint: search for "melting point metals").
|
 |
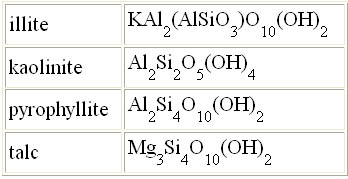
| Question 9: On the right are formulas
for four different clays. What three elements are found in each of these
clays? Also, notice that "talc" is a clay. You know it as the
main ingredient in talcum powder; however, being a clay, talc can be heated
to make porcelain-like dinnerware. They call it steatite porcelain. Sometimes
these formulas are written to show the water, for example, kaolinite is
also written Al2O3·2SiO2·2H2O.
The element count is the same, but you can better see the water (H2O)
that will be driven off upon heating. |
 |
|
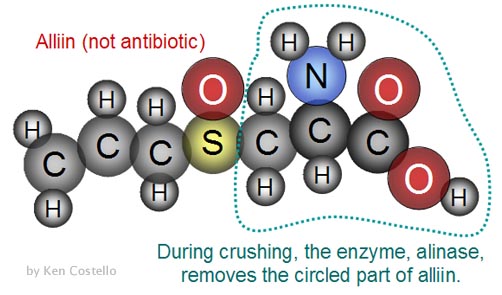
Question 12: Garlic is known for its antibiotic
capabilities. The first structure, alliin, is contained in garlic
but has no antibiotic properties. When crushed, an enzyme (alinase) in
the garlic comes in contact with "alliin" and removes
the right half . Note: C=carbon, H=hydrogen, S=sulfur,
O=oxygen.
|
 |
|
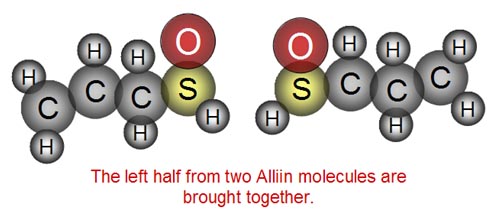
The enzyme takes two halves from two Alliin molecules
and...
|
 |
|
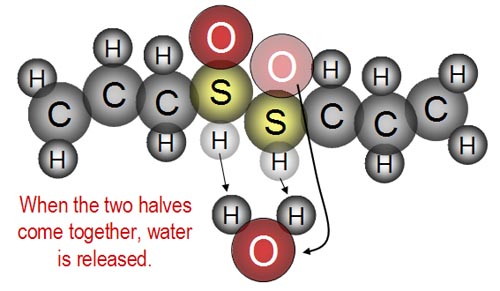
...and as the two halves are joined, water is released.
|
 |
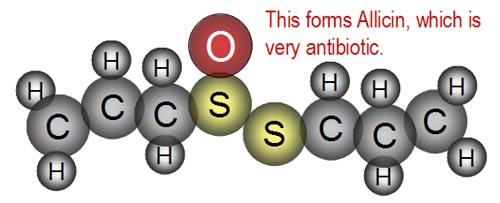
| The result is the compound, "Allicin,"
which is very antibiotic. This compound gives garlic its known antibiotic
properties. |
 |
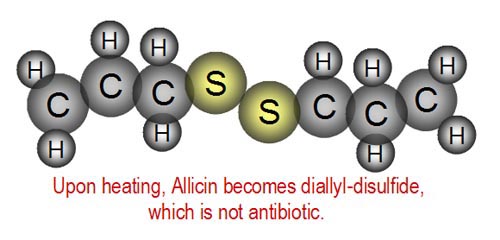
| However, when cooked, the Allicin breaks
down into diallyl-disulfide. Unfortunately, this is not antibiotic.
So garlic in cooked foods is not as healthy. What's the difference in the
molecules of Allicin above and diallyl-disulfide shown to the right? |
 |
| Congratulations on getting through
your first quiz. Send your answers to me at chm130@chemistryland.com |















