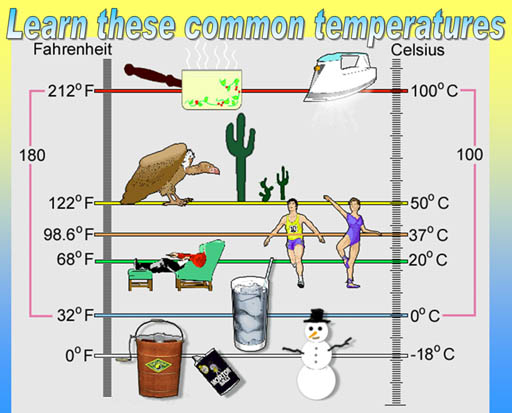
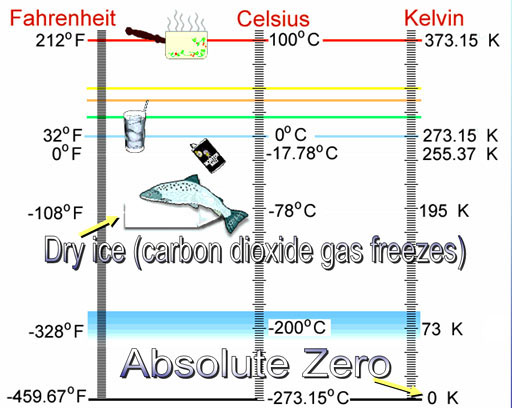
Below is the study guide for the mid-term exam. You have a 3 week window to come into the Testing Center and take the mid-term. Dates are from June 25th to July 16th.
STATES OF MATTER |
| Classical states of matter are Gas, Liquid, Solid. Some non-classical, yet common states of matter, are supercooled liquid (also called glass), liquid crystal, superfluid, and plasma. |
States of Matter |
Examples |
Properties of the State of Matter |
Shape |
Spacing |
Movement of Atoms/Molecules |
Compressible |
Temperature |
| Gas |
Air, helium in balloons, gasoline vapor, etc. |
Expands to fill shape of its container |
Large |
Move at high speeds and random directions |
Yes |
Low to High |
| Liquid |
Liquid water, molten metals, solvents, solutions, liquefied gases, etc. |
Levels itself to fill its container |
Small |
Move at low speeds and random directions |
No |
Low to High |
| Solid |
Metal, rocks, wood, sugar, bone, rubber, etc. |
Shape is fixed |
Small |
Vibrate but don't move |
No |
Extremely low to High |
| Liquid Crystal |
Molecules inside of liquid crystal displays (watches, computer screens, cell phones) |
Levels itself to fill its container |
Small |
Move in one direction but cannot rotate, so stay in semi-crystal formation. |
No |
Moderate temps |
| Superfluid |
A fluid that flows with no friction, so it can climb the walls of its container, e.g., liquid helium. |
Levels itself to fill its container but then climbs out. |
Small |
Move at low speeds and but direction is not always random |
No |
Extremely cold temps |
| Supercritical fluid |
A liquid above a certain temperature and pressure that acts like a gas and liquid. (supercritical CO2 decaffeinates coffee beans) |
Expands to fill its container like gas but has solvent properties like liquids |
Medium |
Move at high speeds and random directions |
Yes |
High |
| Plasma |
Ionized gas. Electrons and ions moving independent of each other in a gas state (neon lights, the Sun) |
Expands to fill shape of its container |
Large |
Move at high speeds and random directions |
Yes |
Very high |
On the exam, some of the cells above will be empty. You will fill those in. In the "Examples" column, you will only need to give one.
|
Four classifications of matter can be determined by asking 3 questions. Those four classifications are heterogeneous mixture, homogeneous mixture, element, or compound.
|
Matter |
|
|
No |
Is it uniform throughout? |
Yes |
|
It's a heterogeneous mixture (like sand and water) |
|
It's homogeneous matter. (well mixed) ↓ |
|
|
No |
Does it have a variable composition?
(more than one formula?) |
Yes |
|
Then it is a pure substance.
↓ |
|
Then it is a homogeneous mixture (like a solution or beverage, or any mixture of two or more substances that are thoroughly mixed) |
No |
Can this pure substance be separated into simpler substances? |
Yes |
|
Then it's an element |
|
Then it's a compound (one or more elements chemically combined) |
|
Using the questions in the above table, determine the classification of the following items: (Choices: heterogeneous mixture, homogeneous mixtures, element, or compound.) Note a heterogeneous mixture has parts that are visibly different to other parts of the mixture. A homogeneous mixture looks the same. A compound will have a formula. For example, there is no formula for orange juice. So it's not a compound. There is a formula for ice, which is H2O. If something is an element, then it will be found on the Periodic Table of the Elements.
Root beer float, sodium chloride (table salt), aluminum foil, grape juice, toothpaste, raisin bread, quartz, syrup, Pepsi on ice, filtered orange juice, orange juice with pulp, air, pure water, muddy water, ice made from pure water, plain paper, Christmas wrapping paper, 24 karat (carat) gold ring, 14 karat gold ring, iron nail, rusty iron nail, glass in a window, urine, NaHCO3 (baking soda). (These are answered at bottom of this study guide with explanations. First see if you can figure them out). |
More Classifications: Atom, Element, Molecule, Compound, Chemical, Substance |
In chemistry sometimes we are referring to a single object and sometimes we referring to a collection of those objects.
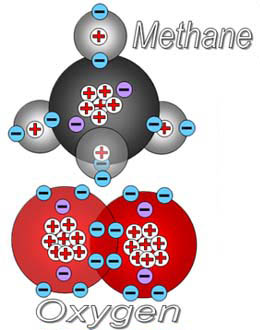
An atom is a single object that consists of a nucleus (protons and neutrons) which is orbited by electrons. It is usually drawn as a sphere (or circle). Also, in formulas like CO2, the "C" represents a carbon atom and "O" is an oxygen atom.
An element is a collection of atoms that have the same number of protons. For example, the element carbon is a collection of carbon atoms which always have 6 protons. Like mentioned earlier, an element is listed in the Periodic Table of the Elements.
A molecule is a single object with two or more atoms (same or different) bonded to each other. A molecule is usually drawn as a group of connected atoms. See below.
 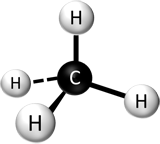 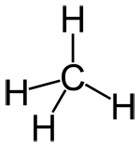
On the left is a molecule of phosphorus atoms. In the middle is methane shown with atoms as spheres. On the right is also a molecule of methane shown just with the element symbols.
A compound is a collection of molecules which have two or more different atoms bonded to each other. For example, the word "propane" or its formula (C3H8) could be referring to a molecule of propane or a collection of propane molecules. It depends on the context. For example, in the statements "Propane is now $3 a gallon." or "C3H8 has a boiling point of -43°F.", "propane" and "C3H8" are referring to a collection of propane molecules, so they are talking about the compound of propane. It can't be a molecule of propane, because you can't get a gallon from one molecule of propane. Also, a single molecule has no boiling point. It takes a collection of molecules to boil. In the below chemical equation, C3H8 is a single molecule of propane because we are counting the atoms in a single molecule of propane to balance the equation.
C3H8 + 3O2 → 3CO2 + 4H2O
A chemical can be an element, compound, or a mixture of elements or compounds. "Chemical" is a catch-all name for most any kind of powder, liquid, or gas. For example, a "chemical bath" means something that is cleaned using some sort of chemical compound or mixture.
A substance is also a general name for any element, compound, or mixture. It's also a catch-all name for any kind of powder, liquid, or gas. For example in "substance abuse" the substance can refer to most any kind of drug or chemical. Substance and chemical are essentially interchangeable in chemistry.
On the mid-term, you will simply be matching the definition to the word.
|
Physical and Chemical Properties |
Physical properties are properties of the substance itself. Physical properties do not mention how the element or compound reacts with another substance. That belongs to chemical properties.
Some physical properties are color, form (gas/liquid/solid), odor, melting point, boiling point, malleable (easy to bend or reshape), crystalline, amorphous (no crystal structure), density, electrical conductivity (good conductor or insulator), index of refraction (how much it bends light), and more. Even without using equipment, you can identify the physical properties of substances such as color, form, taste, and odor. However, taste is not a good idea for checking most chemicals.
Example: Physical properties of a natural diamond:
Color: Can be most any color.
Density: 3.5-3.53 g/cm3
Index of Refraction: 2.418
Hardness: 10 on the Mohs scale |
Chemical properties describe how a substance will react with other substances. For example, saying something is flammable is saying it will react with oxygen. So being flammable is a chemical property.
"Strong acids dissolve metals". This is a general statement of a chemical property for strong acids. Notice that it is mentioning a different substance (metals in this case). Chemical properties tell you how a substance will react with another substance.
"Sodium bicarbonate neutralizes acids." This is a chemical property statement for sodium bicarbonate.
"Citric acid is incompatible with alkaline carbonates and bicarbonate." A statement that uses the word "incompatible" is stating a chemical property. In other words, it is saying that this substance will react with these other substances. It's incompatible because citric acid will no longer stay citric acid if in contact with the other substances mentioned. |
| On the test, list 6 physical properties |
On the test, match the chemical property with the correct substance (Below are the correct matches. On the midterm exam, they will be scrambled. Some of the substances might be different, too.)
| Substance |
Chemical Property |
| Sulfur |
flammable |
| Potassium chlorate (KClO3) |
Oxidizer |
| Lye (NaOH/sodium hydroxide) |
Alkaline/neutralizes acids |
| Powdered iron |
easily rusts |
| Gold |
inert |
| Sugar |
digestible |
|
PHYSICAL AND CHEMICAL CHANGES |
| A physical change is when the physical properties of a substance have changed, but the make up (formula) of the substance does not change. For example, water changing to ice is a physical change. The physical properties of ice (such as density, hardness, index of refraction) are different than those of water. However, the formula for ice (H2O) is the same as for water (H2O). So there was no chemical change only a physical change.
Physical changes are usually easily reversible. |
A chemical change means the substance changes into another substance. In other words, its chemical formula changes. If a substance decomposes, that is a chemical change. If a substance reacts with another substance, that's a chemical change. For example, when the Statue of Liberty was erected in 1886, the copper metal was copper in color. However, by 1900, it had turned a turquoise color. The copper underwent a chemical change, going from metallic copper to a mixture of copper oxides, copper carbonates, copper sulfates, and copper chlorides due to exposure to various chemicals in the air and rain.
Chemical changes are not as easily reversible as physical changes. That's why we worry more about unplanned chemical changes. |
Physical Change Desired: Air Conditioning
The freon in air conditioners (home or car) changes from gas to a liquid and back to a gas, which then repeats. This is a physical change because the freon does not change its chemical makeup, just its state (liquid versus gas).
If freon leaks out, ultraviolet light in sunlight will decompose the freon. One decomposition product is chlorine gas. Is decomposition a chemical or physical change? |
Chemical Change Desired: Combustion for engines and stoves.
Whether it is combustion in the engine of a car or on the stove, combustion requires a chemical change. In these cases, it is a hydrocarbon (compound made from carbon and hydrogen atoms) that is combining with oxygen to form carbon dioxide and water. Energy is released and we get heat to build up pressure in the engine's cylinders or heat to cook our food. A chemical change is desired in this situation because that's what will produce heat.
If liquid gasoline enters the cylinder and simply turns to vapor but does not burn, is that a chemical or physical change? |
Physical Change Desired: Lighting
Most lighting depends on a physical change. The traditional light bulb has a filament that heats up and glows. That is a physical change. When the electricity is off, the filament cools down to its original physical state. However, if the bulb is cracked and air is introduced, the oxygen in the air reacts with the tungsten filament which then undergoes a chemical change as it becomes tungsten oxide, which is brittle. So the filament breaks and the light bulb stops working. In this situation, we don't want a chemical change. So the bulbs are engineered to prevent chemical changes.
Candles produce light. Is that because of a physical change, chemical change or both?
LED lights don't have glass enclosures to keep out oxygen. So the material that makes up LED is resistant to oxygen. Is that a physical property or chemical property? |
Chemical Change Not Desired: Food and Medicine Expiration dates
Why do food and medicine have expiration dates? It's because there is a slow chemical process going on. After the expiration date, a significant portion of the ingredients in medicine may have undergone chemical change and converted to some other chemical. Food has the same problem. Protein, fat, sugar, starches, and vitamins that make up food will chemically break down or react with other ingredients in the food or air. Bacteria will also alter the chemical makeup. So in these situations, spoilage or degradation is occurring because of chemical changes. Preservation of food and medicine is designed to slow down or prevent chemical change.
Chocolate is a food. If it melts, is that a physical change or chemical change?
Does cooking of food cause a physical change or chemical change?
A new bottle of Aspirin doesn't have much of a smell. However, over time it will smell like vinegar. Do you think a physical change or chemical change took place? |
METRIC SYSTEM |
| The English system of measurement has a bewildering array of units. Here are just a few of them:
Pounds, poundals, stones, grains, tons, short tons, long tons, ounces, avoirdupois ounces, troy ounces, apothecary ounces, pennyweights, centals, geepounds, carats, drams, scruples, gammas, long quarters, short quarters, and slugs.
Which of these above units are you familiar with? (no right or wrong answers). |
The Metric system is based on the Earth, water, and 10 fingers. These items we all have access to, so the metric measurements can be recreated by anyone on Earth.
The meter is the distance from the north pole to the equator divided by 10 million. This distance is a little longer than a yard. So we now have a distance measurement
A box that is one tenth of a meter (a decimeter) on all sides is called the liter. So we now have a volume measurement.
A liter of water is assigned the mass of one kilogram (1000 grams). So we now have a mass measurement.
The prefixes of the metric system are based on ten (because we have 10 fingers).
Use the body to know these metric distances
One meter: Distance from neck to tip of fingers.
One meter: Distance between steps in a fast walk or when pacing out distances.
One decimeter=(0.1 meter): Width of the hand.
One centimeter=(0.01 meter): Width of a fingernail.
One millimeter=(0.001 meter): Thickness of a fingernail.
100 micrometers=0.1 millimeters=(0.0001 meter): The smallest object your eyes can see, which would be an object that is the width of a human hair. |
Use these average volumes related to the body to know metric volumes.
One liter: Volume of stomach when it feels full.
Half a liter: Volume of cupped hands.
Half a liter: Volume of air in normal breath.
5 liters: Volume of human head
5 liters: Volume of blood in body
5 milliliters (0.001 liters): Volume of eyeball
90 femtoliters (10-15 liters): Volume of one red blood cell
3 liters: Volume of water ingested per day
1.5 liters: Volume of urine output per day
|
Use these average masses related to the body to know metric masses.
8 grams: Mass of eyeball
65 grams: Mass of tongue
300 grams: Mass of heart
1.5 kilograms: Mass of brain
5 kilograms: Mass of head
100 kilograms (100,000 grams): 5% of American males are above this weight
87 kilograms (87,000 grams): Average weight of American male
3 kilograms: The mass one human hair can hold without breaking.
6,000 kilograms: If the hair on your head was woven into a rope, it could support 6,000 kilograms (the mass of about 3 automobiles.) |
| Regarding these questions about metric sizes that relate to the human body, the test will show the size and you will match it to the part of the body that is approximately that size. |
| The easiest way to do get rid of metric prefixes is to replace the prefix with the amount that prefix represents. For example, to change 54 milliliters to liters, change the "milli" to either 0.001 or 1/1000. So that means 54 x 0.001 or do 54 x 1/1000. Both get 0.054 liters. An easy way to add a metric prefix is to divide by what the prefix represents. For example, changing 5.5 liters to milliliters means divide by 0.001 to get 5,500 milliliters. |
Convert the following metric volumes: (On the test, the amounts will be somewhat different)
3000 milliliters to liters:
5 liters to milliliters:
half liter to milliliters:
1.5 liters to milliliters:
One trillion femtoliters to milliliters: |
Convert the following metric distances: (On the test, the amounts will be different)
2.35 meters into centimeters:
2.35 meters into millimeters:
1 centimeter into millimeters:
2 decimeters into meters:
0.35 meters into decimeter:
505 micrometers into meters:
|
Using Label-Factor Method (also called Dimensional Analysis) for Density problems.
This approach analyzes the dimensions (unit labels) of the problem and sets up a problem so that the starting dimension is changed to the answer by using a conversion fraction that cancels out the dimensions (units) we don't want and leaves (or introduces) the ones we want in the final answer.
|
10.0 mL x 19.3 g = 193 g
1 mL
50.0 g x 1 mL = 2.59 mL
19.3 g |
Here we see milliliters (mL) of gold being converted to grams (g) of gold by using the density of gold (19.3 grams per mL). The mL cancel out leaving just grams.
In the second calculation, we see grams of gold being converted to milliliters of gold by using the density of gold inverted. grams is on the bottom so that they will cancel the starting grams. |
| The below tables show the same calculations setup above. It simply shows it in a spreadsheet layout. The advantage of setting up problems in a spreadsheet is that the computer will recalculate the answer whenever you change any of the values. In other words, if the 50.0 g was changed to 144.6 g, the spreadsheet would display the new mL instantly. This of course depends on the formula placed in cell G1 saying "=A1*D1/D2". Notice the units in red get canceled, and the units in blue remain. Note: Sometimes column "C" with the "x" is left out, but it is still understood that multiplication of the fractions is taking place. Spreadsheets also let us use the row number and column letter to identify a particular cell. For example, A1 and B1 show the starting quantities. |
|
A |
B |
C |
D |
E |
F |
G |
H |
1 |
10.0 |
mL |
x |
19.3 |
g |
= |
193 |
grams |
2 |
|
|
|
1 |
mL |
|
|
|
|
A |
B |
C |
D |
E |
F |
G |
H |
1 |
50.0 |
g |
x |
1 |
mL |
= |
2.59 |
mL |
2 |
|
|
|
19.3 |
g |
|
|
|
The below table is the same as the above without the times "x" column.
|
A |
B |
C |
D |
E |
F |
G |
1 |
50.0 |
g |
1 |
mL |
= |
2.59 |
mL |
2 |
|
|
19.3 |
g |
|
|
|
|
| The below dimensional analysis setup (label-factor method) is similar to a problem in the tutorial. This setup converts 2 miles per hour into centimeters per second. Each conversion fraction have numerators and denominators that are equal to each other. These are written to cancel out the units we don't want (miles and hours) and get us to the units we do want (centimeters and seconds). Just like multiplying fractions, you multiply all of the numerators and divide by each of the denominators. You can skip any values of "1" because that doesn't change the quantity. So multiply 2 x 440 x 36 x 2.54 to get the numerator. Then divide by 0.25 followed by division by 60, then divide by the other 60. |
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
1 |
2 |
miles |
440 |
yards |
36 |
inches |
2.54 |
cm |
1 |
hr |
1 |
min |
= |
? |
cm |
2 |
|
hr |
0.25 |
mile |
1 |
yard |
1 |
inch |
60 |
min |
60 |
sec |
|
|
sec |
|
On the midterm exam, there will be some dimensional analysis problems that are set up that you solve. Some will be fully setup (like above) and some will be partially set up like below.
Driving 424 miles in a car that gets 22.0 miles per gallon will take how many liters of gas? The dimensional analysis is set up below to solve that, but you need to write the correct dimensions (measurement units) in their proper place. B1 shows that miles is the starting units. The information of 22.0 miles per gallon is inverted so that miles is in the denominator and cancels the miles in B1. At column E we have volume in gallons, but the question wants it in liters. So columns G and H convert the gallons to liters. So you would write "miles" in E2, "liters" in H1, and "gallons" in H2. Do the arithmetic to find the quantity of liters that is written in S1. On the exam, some units will be left out for you to insert into the proper cell. |
|
A |
B |
C |
D |
|
F |
G |
H |
I |
1 |
424 |
miles |
1 |
gallon |
3.785 |
liters |
= |
______ |
liters |
2 |
|
|
22.0 |
miles |
1 |
gallon |
|
|
|
|
| Ethanol is a common laboratory solvent and has a density of 0.789 g/mL. What is the mass, in grams, of 125 mL of ethanol? (Below is the partial setup to solve this problem. You will need to write the correct value and units in C1 and D1. Also, calculate F1. |
|
A |
B |
C |
D |
|
F |
G |
1 |
125 |
mL |
________ |
_______ |
= |
______ |
grams |
2 |
|
|
1 |
mL |
|
|
|
|
| Let's say you want to know the volume (in liters) of the iron in a car that gets crushed and melted down. The iron in the car weighs 3,155 lbs. Iron's density is 7.874 g/mL. The below dimensional analysis spreadsheet is setup. In the example below all the fractions are setup. On the exam, some data will be left out. You should memorize how many lbs equal a kilogram. Do the arithmetic to find the quantity of liters (J2). Notice how D3 cancels B2. Notice E3 cancels the "k" in "kg" in D2. The strategy of dimensional analysis (label-factor method) is to let the units guide how you set up and solve these kind of problems. Notice that the density is inverted so that grams is on the bottom so that it cancels the "g" in "kg" in D2. "mL" is on the top because we want the answer in "Liters". On the midterm exam, you will have to decide where to place the measurements of density to get the answer. For example, the red text will not be there. |
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
1 |
Mass of iron |
convert lbs to kg |
cancel k |
Use density to convert grams to mL |
cancel milli |
|
|
|
2 |
3155 |
lbs |
1 |
kg |
1000 |
1 |
mL |
0.001 |
= |
______ |
liters |
3 |
|
|
2.205 |
lbs |
kilo |
7.874 |
grams |
milli |
|
|
|
|
| It's a sad fact but the United States is about the last country not to convert completely to metrics. This always adds some extra conversions in chemistry. For example, in a brewery the recipe calls for a concentration of malt of 2.5 grams malt per 100mL of brew in a vat that holds 1,500 gallons of brew. How many 25-lb bags of malt is needed? (equalities: 1 gallon=3785 milliliters, 1 lb=454g) Note the dimensional analysis below. It begins with the concentration, which is mass per volume. That it multiplied by the volume of the vat. Normally, that is all that is needed to find mass because the volume cancels. However, that only works if volumes have the same units and the mass is the unit you want. Unfortunately, our units for volume don't match (mL vs. gallons). Also grams is not the weight we want. We want the weight in the number of 25-pound bags. See below dimensional analysis that shows how to solve this. On the test, you will fill in columns G and H and solve for L2 (number of bags). |
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
1 |
concentration of malt |
Concentration x vol=mass |
Convert gal to mL |
convert g to lb |
|
|
|
|
|
2 |
2.5 |
g |
1500. |
gallon |
3785 |
mL |
1 |
lb |
1 |
bag (25lb) |
= |
______ |
25-lb bags |
3 |
100 |
mL |
|
|
1 |
gallon |
454 |
grams |
25 |
lbs |
|
|
|
The grams in B2 is canceled by what cell?
The mL in B3 is canceled by what cell? |
ENERGY |
 |
In the tutorial on energy, I said we can think of just two types of energy: Energy doing work, and energy waiting to do work. When it's doing work, we see it moving something, giving off some form of light, or changing the temperature of something like this train is doing. Work energy is also defined as a force applied to something which moves it across a distance. Like the force of lifting a brick from one height to a higher height. Or the force of a car pushing against the air as it moves down the highway. |
|
| Energy that is waiting to do work (has to the potential for work) is called potential energy and is recognized in 3 ways: |
| 1) It sits above ground level (like a lake behind a dam that sits higher than the land below the dam.) |
2) Something is compressed or stretched in someway (like compressed air or a compressed or stretched spring). |
3) Crowding of like charges or a separation of unlike charges (like a battery, capacitor, or electrons and protons on the same or different atoms. |
|

|
|
| Kinetic energy is the energy of something that moving. However, if that moving object is not pushing anything (like a spinning flywheel, a swinging hammer, or meteor flying through space), then this energy is just waiting to do work. So this energy is similar to potential energy yet it's not normally classified as potential energy. When these moving objects start to push on something, then they do work. Again, when that object moves freely then the kinetic energy is waiting to do work. It's like potential energy in that sense that it has the potential to do work but hasn't done it yet. Again, when the moving object pushes on something and gets it to move. Then it becomes work energy, which is a force times the distance that force is applied. |
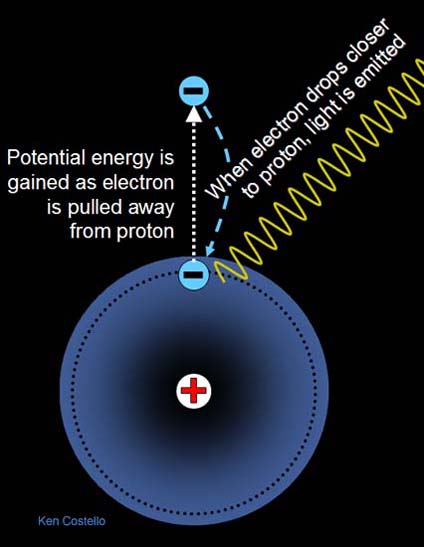 |
Chemical energy is a type of potential energy. It's similar to raising objects against gravity except instead of moving objects against the attraction of gravity, it is moving objects against the attraction of plus and minus charges. To illustrate this, let's look at the simplest of elements, hydrogen....
The hydrogen atom consists of one proton (+) and one electron (-). The proton sits in the center (nucleus) of the atom. The diagram shows the electron as a particle orbiting the nucleus. If an electron is moved farther away from the proton, it takes energy because they are attracted to each other (like gravity). The farther away the electron has been moved away from the proton, the more energy it takes, and the more potential energy stored in the electron. When the electron drops back closer to the proton, it will convert that potential energy to light energy. The light energy can be in the form of infrared, visible, ultraviolet, or even X-ray light.
Helium has two protons. Would you think it takes more or less energy to pull an electron away from the helium nucleus compared to a hydrogen nucleus?
Would an electron that has been pulled away from a helium nucleus have more or less potential energy than an electron that was pulled away from an hydrogen nucleus? |
| Check which items below possess kinetic energy |
Check which situations below are exhibiting energy doing work. |
[ ] Roller coaster stopped at the top of a hill.
[ ] A stretched rubber band
[ ] A candy bar
[ ] Water behind a dam
[ ] Wind
[ ] A car coasting down a hill
[ ] A car going up a hill |
[ ] Blowing up a balloon.
[ ] A car just sitting in the driveway.
[ ] A stick of dynamite.
[ ] Dynamite exploding.
[ ] Earth going around the sun.
[ ] Wind pushing on wind generators.
[ ] Talking (See end of study guide for answers)
|
|
| Horsepower is work energy per minute. It came from the fact that one horse using a pulley could lift 330 lbs 100 feet in one minute. So that's 330lbs x 100 ft / 1 min. Or 33,000 ft·lbs/min. In other words, the pounds (lbs) times the feet divided by the minutes will come out to 33000 if one horsepower. We need an elevator to lift 1550 lbs. If this elevator gets 1550 lbs up to the 20th floor (200. feet) within half a minute, what horsepower motor is it using? Dimensional analysis uses the trick of letting the dimensions guide how to do the problem. Since horsepower has the units of ft·lbs/min, then it says to multiply the feet by the pounds, then divide by the minutes. On the exam, the values will be different and the problem won't be totally set up.
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
1 |
Distance lifted |
force to lift |
Divide by minutes |
convert to horsepower |
|
Answer in horsepower |
2 |
200. |
ft |
1550 |
lbs |
|
|
1 |
horsepower |
= |
______ |
horsepower |
3 |
|
|
|
|
0.50 |
min |
33000 |
ft·lbs/min |
|
|
|
ANSWERS:
|
Heterogeneous mixtures: Root beer float, raisin bread, muddy water, Pepsi on ice, orange juice with pulp, Christmas wrapping paper, rusty iron nail.
With heterogeneous mixtures you see at least two different components. Imagine taking a small sample from these. If it is heterogeneous, it is likely that the sample you take will not represent the whole mixture. For example, in the root beer float, you may sample the ice cream at the top and say this is made from dairy products. However, if you sample the root beer part, then you would see no dairy products. A heterogeneous mixture is hard to analyze because different parts of it will have different compositions. Christmas paper is unlike plain paper because it has colored patterns. If you were to sample part of the paper that was red, it would have a different composition than a part of the paper that was green.
Homogeneous mixtures: Grape juice, toothpaste, syrup, filtered orange juice, plain paper, 14 karat gold ring, glass in a window, urine, air.
These homogeneous mixtures will look uniform; however, you know from experience that they are not pure. For example, corn syrup is clear like water, but you know syrup is a mixture of sugar and water. A 14 karat gold ring is a mixture of gold with some other metal. Since 24 karat is 100% gold, 14 karat is 14/24 (58%) gold. It's homogeneous because the gold is evenly mixed with the other metal. Homogeneous mixtures are good to analyze because if you take a small sample of the mixture, you will get results that represent the whole mixture.
Compounds: Sodium chloride (table salt), ice made from pure water, NaHCO3 (baking soda), quartz, pure water
Compounds all have a single formula. Mixtures don't have one formula because they are made from two or more compounds. Quarts has the formula SiO2. If a chemical name is given such as sodium carbonate, lead(IV) sulfate, dinitrogen monoxide, or iron(II) chloride, then these are compounds. Each has a specific formula.
Elements: Aluminum foil, 24 karat (carat) gold ring, iron nail
Elements are all listed on the Periodic Table. The table doesn't say "aluminum foil" but it does say Aluminum, which is what aluminum foil is made of. An iron nail is simply iron. A 24 karat gold ring is pure gold. Gold is an element. A 14 karat gold ring is not pure gold, so a 14 karat gold ring is not an element.
|
| More ANSWERS: Atomic theory, Atomic mass, Molar mass |
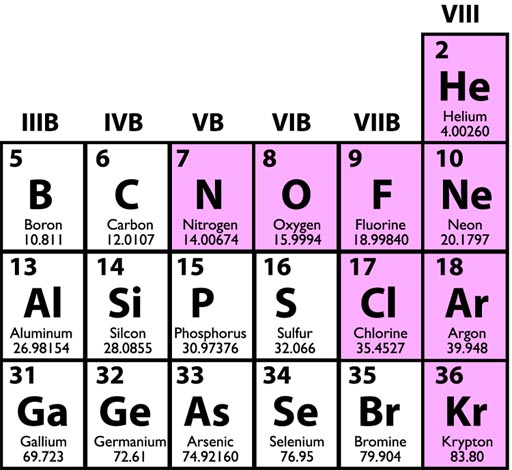 |
Using the portion of the Periodic Table on the left, answer the below questions:
One mole of helium atoms weighs how many grams? 4.00260 g. (4 g is OK)
One mole of oxygen atoms weighs how many grams? 15.9994 g (16 g is OK)
One mole of oxygen molecules (O2) weighs many grams? 15.9994 g x 2 = 31.9988 g (32g is OK)
One mole of CO2 (carbon dioxide) molecules weighs many grams? 44.0095 g (44 g is OK)
What element has the atomic number of 33? Arsenic
How many protons does an atom of aluminum have? 13
How many electrons does a neutral atom of boron have? 5
Why are some of these elements shown with a pinkish background? Those are gases at room temperature.
What two elements are most chemically similar to chlorine? Fluorine and bromine
Above fluorine (F) are the letters "VIIB". What is significance of the Roman numeral VII? The elements in this column have 7 (VII) electrons in their outer shell.
Fluorine atoms have the average atomic mass of 18.99840. Therefore, how many neutrons do most fluorine atoms have? This number is close to 19. So with fluorine having 9 protons, those atoms must have 10 neutrons to have an atomic mass of 19.
If the nucleus of a silicon atom were to gain a proton, what element would it become? Phosphorus.
If the nucleus of a carbon atom were to fuse with the nucleus of helium, what element would they become? Oxygen
A mole of krypton gas is how many krypton atoms? 6.022x1023 atoms.
20.1797 grams of neon gas is how many moles of neon gas? 1 mole
50 grams of carbon is how many moles of carbon? 50 g * 1 mole per 12.0107 g = 4.16 moles (or 4 moles)
|
More ANSWERS:
Check which items below possess kinetic energy |
Check which situations below are exhibiting energy doing work. |
[ ] Roller coaster stopped at the top of a hill.
[ ] A stretched rubber band
[ ] A candy bar
[ ] Water behind a dam
[X] Wind
[X] A car coasting down a hill
[X] A car going up a hill |
[X] Blowing up a balloon.
[ ] A car just sitting in the driveway.
[ ] A stick of dynamite.
[X] Dynamite exploding.
[ ] Earth going around the sun.
[X] Wind pushing on wind generators.
[X] Talking
|
Last semester, the grades on the mid-term were curved so that scores that normally got a B, C, or D, were given an A, B, and C respectively. It's likely the same curve will be done this summer session. For example, if you got 70%, that normally is a C, but I gave an extra 10% to all scores, making the score 80%, which is a "B".
←CHM130 Home page |