Page 80: Objectives
1. Classify compounds according to their behavior in dilute water solution.
2. Observe heat of solution.
3. Prepare and use a solubility curve.
A. Electrolytes
An electrolyte is a substance that produces ions in solution. The solution of an electrolyte has electrical conductivity...
The word, "Electrolyte" should be explained more. "-lyte" is from a Greek word, Lytos, meaning loosened or soluble. Electrolytes are soluble in water meaning they are pulled apart (loosened) by the water. Remember, water has partial negative and partial positive charges. "-lyte" also indicates that this is the substance that can undergo "Electrolysis". "Lysis" means about the same thing. It is the pulling apart or decomposing of something. Electrolysis means the breaking apart of something using electricity. Remember the lab where water underwent electrolysis and the water was decomposed into hydrogen and oxygen gases. Electrolytes are vulnerable to electrolysis. Put them in water and attach wires from a 9 volt battery and they will decompose. Copper (II) chloride (CuCl2) put in water will dissolve into copper ions and chloride ions. Let the water evaporate and you back the copper chloride. However, expose it to a battery and the negative chlorine ions will be attracted to the positive side of the battery and the ions turn into chlorine gas and bubble away. The positive copper ions will be attracted to the negative side of the battery and will plate out on that wire as copper metal. So the copper chloride was decomposed.
In this lab we are not really decomposing the electrolytes using electrolysis. We are just dissolving them in water. (Not as much fun but easier to do). If a substance dissolves in water, it gives you insight as to the make up of the that substance. In other words it tells you that there are charges or partial charges on the substance that water is pulling on. If the solution conducts electricity, it tells you more. It says that the substance has split apart into ions. Remember chemistry has the disadvantage of trying to study things that are too small to see. So we depend on these indirect methods of "looking at" the substances. For example, table salt (NaCl) in water conducts electricity, so we can "see" the sodium ions (Na+) moving independent of the chloride ions (Cl-).
Page 82: Exp 11
A. Electrolytes
| Substance | Ionic, Polar, or Nonpolar | Non-electrolyte | Weak electrolyte | Strong electrolyte | Ions in solution (Formulas and charges) |
| Distilled water | Polar | X | Essentially none | ||
| Tap water | Ionic | X (because few ions) | Na+, Cl-, Ca2+, CO32- | ||
| Solid sugar | Polar | X | none | ||
| Sugar solution | Polar | X | none | ||
| Solid NaCl | Ionic | X | Not applicable | ||
| NaCl solution | Ionic | X | Na+, Cl- | ||
| Glacial HC2H3O2 | Polar | X | none | ||
| Diluted HC2H3O2 | Ionic | X | H+, C2H3O2- | ||
| 1M NH4OH | Ionic | X | NH4+, OH- | ||
| 1M NH4OH + diluted HC2H3O2 | Ionic | X | NH4+, C2H3O2- | ||
| 1M NaOH | Ionic | X | Na+, OH- | ||
| 1M HCl | Ionic | X | H+, Cl- | ||
| 0.1M CuSO4 | Ionic | X | Cu2+, SO42- |
| Name | Formula | Observed temperature change | Classify solution process as exothermic, endothermic, neither |
| Ammonium chloride | NH4Cl | endothermic | |
| Lithium chloride | LiCl | exothermic | |
| Anhydrous sodium sulfate | Na2SO4 | ? exothermic in observed temp increase, endothermic if cooling | |
| Potassium bromide | KBr | ? exothermic in observed temp increase, endothermic if cooling |
3. Solubility Curve
| Mass of dish + solution | 35.238 g |
Solution temperature 10 C |
| Mass of empty dish | 29.865 g |
Calculations 2.004 g KBr = 0.3730 = 37.30%
|
| Mass of solution | 5.373 g |
|
| Mass of dish + dry KBr | 31.869 g |
|
| Mass of empty dish | 29.865 g |
|
| Mass of dry KBr | 2.004 g |
|
B. Solubility tables and graphs are usually prepared using the number of grams of solute dissolved in 100g of solvent. [Note: Above it was 100 grams of solution (solute=KBr+ solvent=H2O) . In these tables, they want "per 100 grams" of just water (solvent). ] This can be calculated from %KBr.
Example: For a 21% KBr solution, 100 g of solution contains 21 g of KBr and 79 g of water. This gives us 21 g KBr per 79 g of water.
To find g KBr per 100g of water:
21 g KBr ÷ 79 = 0.2658 g KBr x 100 = 26.58 g KBr
79 g H2O ÷ 79 1 g H2O 100 100 g H2O
Or you could leave off the dividing step and that math would be 21/79 x 100. The bottom 100 does not get divided because we want to end up with per 100:
21 g KBr x 100 = 26.58 g KBr
79 g H2O 100 100 g H2O
Temperature C |
%KBr |
Calculations |
g KBr/100g H2O |
10 |
37.3 |
100 g H2O x 37.3 g KBr 62.7 g H2O |
59.5 |
30 |
41.4 |
100 g H2O x 41.4 g KBr 58.6 g H2O |
70.7 |
40 |
43.0 |
100 g H2O x 43.0 g KBr 57.0 g H2O |
75.4 |
50 |
44.5 |
100 g H2O x 44.5 g KBr 55.5 g H2O |
80.2 |
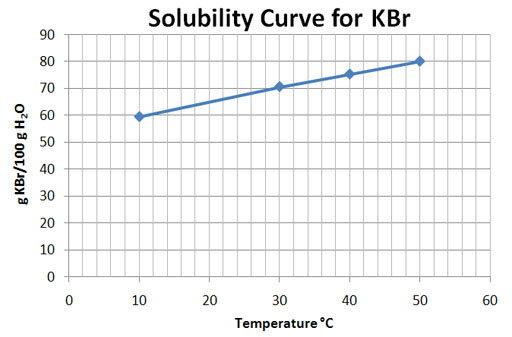
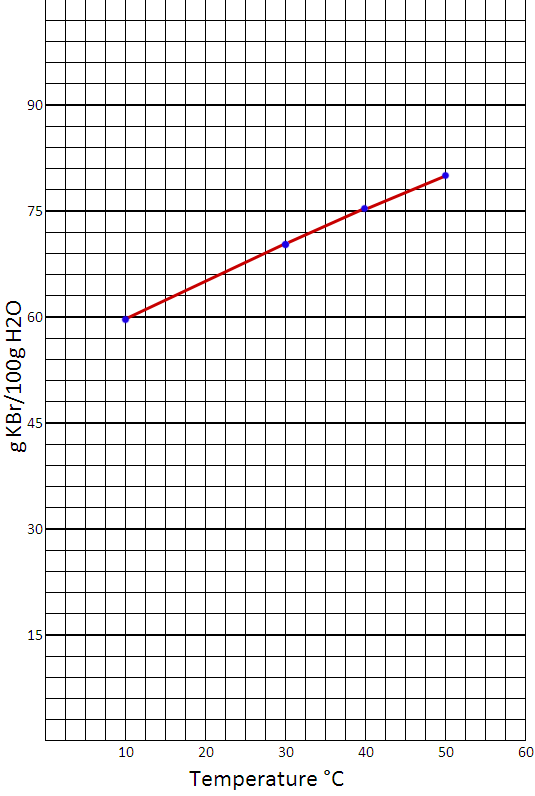
For the vertical axis, I made each square an increment of 3. That way every five squares would be 15. For the horizontal axis, I made each square an increment of 2.5 so that every 4 squares equaled 10.
Exp 11: page 85
Questions
1. Classify each of the following as an electrolyte or nonelectrolyte
a) solid Ca(NO3)2 nonelectrolyte
An electrolyte has to have mobile ions. Any solid will not be an electrolyte. If the solid is melted and ions can move, then it becomes an electrolyte. If this solid is dissolved in water, it will have mobile ions, so then it also becomes an electrolyte.
b) rubbing alcohol (C3H8O) ???
Alcohols normally don't form any ions so it won't conduct electricity. So what is it?
c) H2O nonelectrolyte
You might think of water as an electrolyte because water dissolve electrolytes. However, there are only a few ions formed in pure water. At a neutral pH of 7, that means water has a 10-7 (1 ten millionth) molar concentration of H+ and OH-. But at one ten millionth mole per liter, the number of ions are so small that the conduction of electricity is almost zero.
d) KCl solution ???
KCl dissolves in water and completely dissociates into K+ and Cl-. Those ions are mobile and free to conduct electricity, which means the K+ will travel to the negative wire and the Cl- will travel to the positive wire. They will either pick up or drop off electrons which keeps the current going.
2. Determine if a mixture of equal amounts of 1M H2SO4 and 1M Ba(OH)2 will give an electrolyte or a nonelectrolyte (hint: check the solubility on page 67).
Both of these are obvious electrolytes because sulfuric acid breaks up into H+ ions and SO42- ions. Barium hydroxide forms Ba2+ ions and OH- ions. However, because these are equal amounts of equal concentration (1M each), they will neutralize each other completely. The reaction is:
H2SO4(aq) + Ba(OH)2(aq) --> HOH(l) + BaSO4(s)
or
2H+(aq) + SO42-(aq) + Ba2+(aq) + 2OH-(aq) --> HOH(l) + BaSO4(s)
Since barium sulfate is not soluble, it forms a precipitate (solid). So that doesn't provide any ions for conduction of electricity. The other product is water and pure water doesn't conduct electricity. So the final solution is a nonelectrolyte. If there were unequal amounts of sulfuric acid and barium hydroxide, then there would be some ions left over and the solution would be an electrolyte. But since they said equal amounts and equal concentrations, then no ions are left over.
3. Based on the following dissociation equations, identify which one is a strong acid, HA or HB.
HA < --> H+(aq) + A-(aq)
HB > H+(aq) + B-(aq)
A strong acid will dissociate into H+ ions and the accompanying negative ions. The longer bottom arrow of the HA equation shows that this compound mostly wants to exist as HA, meaning the H+ wants to stay attached to A-. The small arrow on top means that only a small amount of HA will dissociate into H+ and A-. The HB equation has one arrow that points to the right. So HB completely breaks into H+ and B-.
These would be the ones that are endothermic (absorbs energy) when they dissolve in water. So the ones where it felt cooler or the thermometer showed a drop in temperature are the ones on this list
5. From the solubility information in the table below, prepare a solubility curve (graph) for BaCl2.
Temperature |
0°C |
20°C |
40°C |
60°C |
80°C |
100°C |
g BaCl2/100 g H2O |
31.6 |
35.7 |
40.7 |
46.6 |
52.6 |
58.8 |
The graph is 35 squares tall and 24 squares wide. We needed to go to almost 60 in the grams of BaCl2. So with each square incrementing by 2, that allows a height of 70. So that's works. The width needs to go to 100 C. With 24 squares, 4 degrees per square would only be 88 degrees, so I went with 5 degrees per square giving us (5x24=120 degree range).
The line is basically straight but solubility does not need to rise in a linear (straight-line) fashion. So a curve is OK.
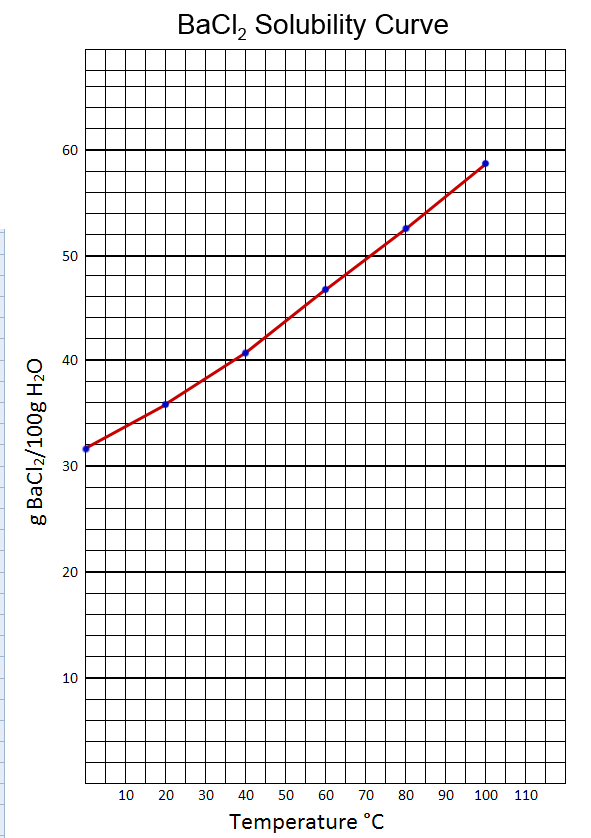
5.
a.) As the temperature increases, does the solubility of BaCl2 in water increase or decrease?
That graph above shows that it increases. This is normal for most soluble compounds. The higher the temperature means the water molecules are moving faster. So they have more energy to break the solid apart. Also, the atoms or polyatomic ions in the solid are vibrating more so it's easier to get them to break loose from each other.
One compound mentioned earlier is sodium sulfate. It's solubility curve is rather strange. It starts out normal with the solubility going up as the temperature goes up, but around 32 degrees C, it actually gets less soluble as the temperature goes up.

According to our BaCl2 solubility graph and data, 52.6 grams of BaCl2 should be in the solution at 80°C. Refrigerator temperatures are around 40°F or 4°C. Looking at the graph, we can estimate that about 32.5 grams of BaCl2 will dissolve in 4°C water. So that means about 20 grams of the BaCl2 will have precipitated from the solution forming a solid at the bottom. Even without looking at the data or graph, when a saturated solution is cooled, some of the dissolved solute will come out of solution. If it doesn't come out, the solution is called supersaturated, which means it is holding more of the solute than it normally can. In those cases a few crystals of that compound will cause a quick crystallization of the excess solute in the solution.