Tutorial and Practice Exam for CHM130 On-campus Final Exam
For the online CHM130 class of Quinn Thacker & Loree Cantrell-Briggs.
Written by Ken Costello (course designer/instructor) |
 |
History: About 11 years ago I created a portable chemistry kit for CHM107. My motivation for doing it was partly because I think the average person should take advantage of the tools of science. I also hope to achieve what happened with computer science. When I went to college, no one had a computer at home, so computer literacy was very low. However, when computers got smaller and less expensive, it was possible to own a computer and take advantage of everything it could do. When people had access to it, they became computer literate and could do many things that normally was not done at home. If our society is going to become science literate, we have to have the tools of science at our disposal. The first step is to know what tools are available. |
 |
MEASURING BODY TEMPERATURE: In the last few years I've noticed a lot more medical devices and medical tests that can be done at home. That's a good opportunity to monitor health needs at home and to learn more about the science behind these devices and tests. So I've designed this on-campus exam to relate to this development.
Many of you are going into a health care field, so it would be relevant to your career. Even if you aren't going into a health field, knowing this is still useful. Below is a tutorial about various tests and devices that are now available for the home. The exam will ask questions about what you learn below plus there will be some questions from the online exam.
Toward the bottom of this page is a practice exam, almost the same as what you will see when you come to the campus to take the on-campus exam. |
 |
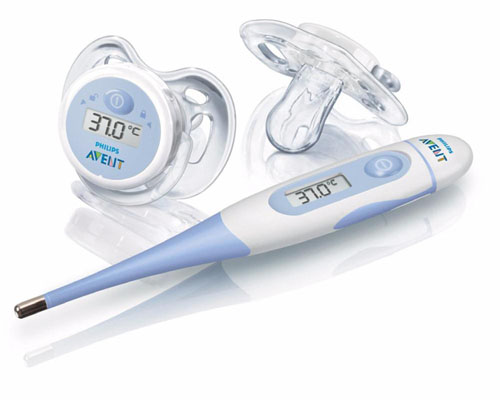
In the past, about the only medical device at home was the glass mercury thermometer. This one was specifically made for measuring a person's temperature. It did a good job if you shook the mercury down to the bottom before using. However, after realizing how unsafe these were, you can't really find these in the stores anymore. The digital thermometers are now popular. There are also many variations of these. |
 |
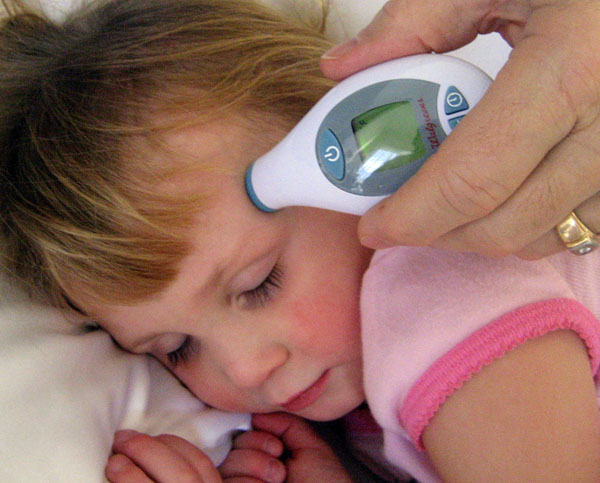
The left digital thermometer is the replacement for the glass mercury thermometers. As electronics got more miniaturized and sophisticated, devices like these have gotten cheaper and common place. The right thermometer is for getting temperature from the ear.
The science behind the left thermometer is that contains a thermistor, which is an electrical resistor that changes its resistance at different temperatures. The name thermistor comes from "thermally" sensitive "resistor". The elements that make up this resistor are often a powdered mixture of manganese oxides, cobalt oxides, nickel oxides and copper oxides. Silicon and germanium are also sometimes added. As the temperature goes up, the electrical resistance comes down and that is converted to a temperature reading on the display.
The ear thermometer (on the right) doesn't contact the ear drum, so it is measuring the amount of infrared light emitting from the ear. The detector is an array of small thermocouples. A thermocouple is two different metals sandwiched together. One type of thermocouple is used by your gas water heater or gas oven to sense if the pilot light is on. It may also used for the thermostat of your air conditioning. These infrared detectors use an array of very small thermocouples (called a thermopile). The metals that are selected to be sandwiched together to make these thermocouples can be chromium, nickel, copper, tungsten, and platinum. As they are exposed to heat, they generate an electrical voltage, which can be converted to a reading on the display window. |
 |
This digital thermometer measures the room temperature and can take readings of the ear or forehead.
The ear and forehead measure the amount of infrared light (heat radiation) coming from the ear drum or the skin. |
|
This seemed like a novel idea. A thermometer that is also a pacifier. It's a good way to monitor a sick baby's temperature. |
|
I've seen infrared based thermometers like in the picture used to measure the temperature of a wide variety of items at a distance. However, this company is selling it for taking your child's temperature. I guess if you can't get you child to sit still, you can take it while they run by. Notice it has a laser dot to show where you are aiming and the temperature of that spot. Actually, I'm a little suspicious of this use of the infrared thermometer. |
 |
MEASURING BLOOD OXYGEN LEVELS AND PULSE: A couple of years ago my mother had pneumonia. Even after she got over the pneumonia, she would often complain about not being able to breath. We weren't sure if this was just psychological or real. In the hospital I noticed they used a device called the Pulse Oximeter to measure her pulse and oxygen levels. I searched Craigslist and found a used portable Pulse Oximeter. It cost $200, but was very useful in discovering that her oxygen levels were truly very low. This prompted the doctor to prescribe a portable oxygen concentrator that gave her pure oxygen when she needed it.
I noticed recently that the drug stores are carrying these small pulse oximeters for about $50. I purchased one for a friend's grandson who has Valley Fever, which is a fungal disease of the lungs.
The science behind these devices is the use of an LED light and a photodiode (a light detector) that looks at the level of two forms of hemoglobin. Hemoglobin is the protein in the red blood cells that carries oxygen. When oxygen is attached to hemoglobin, it's then called oxyhemoglobin (logical). When oxygen has been removed, it's called deoxyhemoglobin. The wavelength of light from the LEDs is set to measure these two forms of hemoglobin.
On the label, you see SpO2%. The "S" in "SpO2% means it's measuring the percent of the hemoglobin that is saturated with oxygen. The "p" in SpO2% means it is measuring the hemoglobin in the peripheral parts of the body (the hands, toes, or ear lobes). The person here shows that 98% of his hemoglobin protein is saturated with oxygen. 97% to 99% is good. Even though 90% is common. |
|
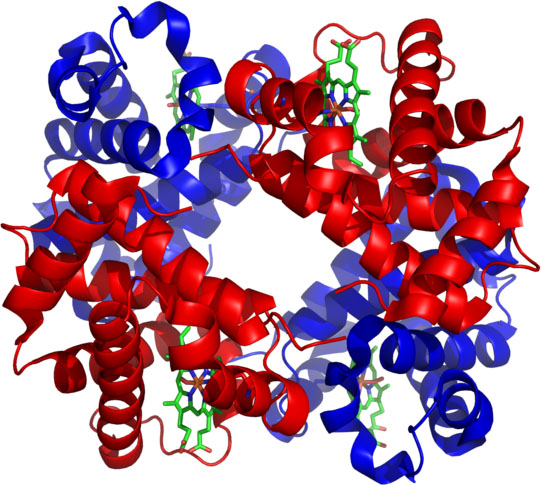
The image is a representation of the hemoglobin protein. The ribbon looking structures represent chains of amino acids because they often twist into ribbon shapes.
The green structures seen are called hemes. These are the structures that hold onto oxygen and carbon dioxide. The heme structures are why the whole protein is called hemoglobin. |
|
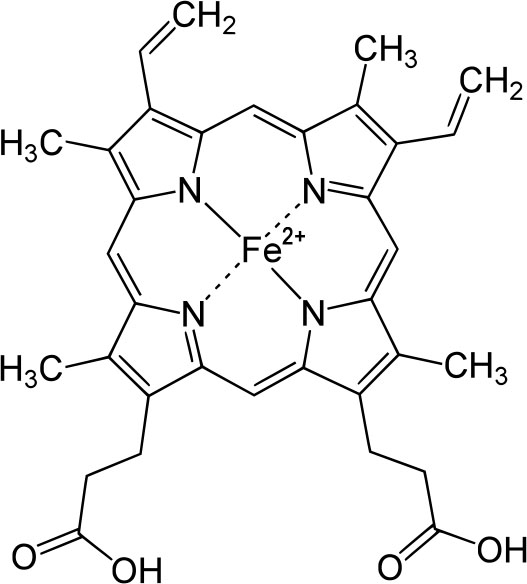
This is the structure of one of the heme groups in hemoglobin. The iron ion (Fe2+) is responsible for carrying the oxygen to the cells and carrying carbon dioxide (waste product) from the cells to the lungs. |
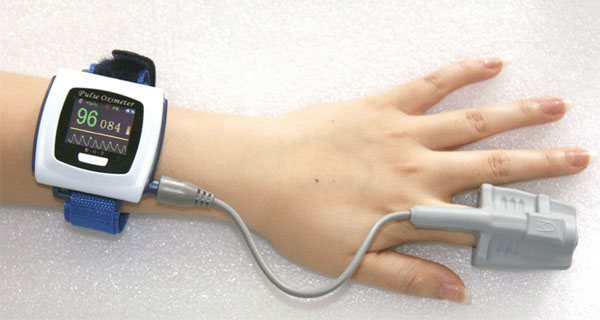 |
Because of the heart beat, the level of hemoglobin in capillaries in the fingers are expanding and contracting with each heart beat. The pulse oximeter picks up this change and displays the pulse rate. For this wrist version, you can see the wave which is the changing levels of hemoglobin from which the pulse can be calculated.
Besides useful for people who have breathing problems, these are being used by pilots and mountain climbers who are at altitudes where oxygen levels are low and monitoring their oxygen saturation would be wise. |
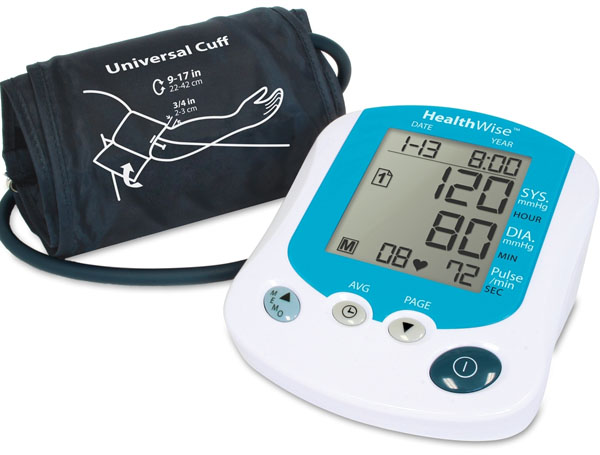 |
MEASURING BLOOD PRESSURE: My father had high blood pressure for many years. It eventually led to him having a stroke 6 years ago. Using a blood pressure monitor like the one in the picture could have warned him that his blood pressure was too high despite the medication he was taking. That might have saved him the devastating effects from the stroke that he still suffers with. After his stroke, the doctors wanted the blood pressure tightly monitored. So we bought one of these automatic blood pressure monitors and checked his blood pressure about 3 times a day. These devices pump up the cuff automatically and take the readings.
When a nurses takes someone's pulse, they pump up the cuff to the point where they can't hear the pulse. Then they go about 30 more millimeters of mercury in pressure. Then they let the pressure slowly drop until the pulse is first heard. That's the pressure where heart beats, which is the maximum pressure. They call that the systolic pressure. Systole is from the Greek word, systole, meaning contraction. As the pressure on the cuff falls, the nurse will continue to hear the sound of blood flowing because the cuff is restricting the blood flow. At the point where they stop hearing any sound, that's marked as the diastolic pressure. That's the pressure when the heart is resting. These automatic blood pressure monitors aren't listening for the pulse, but they are sensing the oscillation (pulsing) that the blood veins cause when the heart beats.
|
 |
This is a blood pressure monitor that has the readout on the cuff itself. So there's no tubing to contend with. These are often placed on the wrist but not always as accurate as those placed on the upper arm.
The low reading is called the diastolic pressure, which is the pressure when the heart is as rest.
Notice the labels of "mmHg". That stands for millimeters of mercury. In chemistry we also measure pressure in millimeters of mercury, but usually call that "torr" after the Italian scientist, Evangelista Torricelli. mmHg and torr are essentially the same. 760 torr=760 mm of mercury. Chemsitry also uses the pressure of atmospheres (1 atmosphere=760 mmHg) or pounds per square inch (14.7 psi = 760 mmHg). |
|
The original and best blood pressure gauges used tubes of real mercury. A pressure of 126 mmHg meant the person's heart beat had the pressure to raise the mercury in the tube to a height of 126 millimeters (about 5 inches).
The technical name of these blood pressure gauges are sphygmomanometer. "Sphygmo-" means "pulse" and "manometer" means "pressure measuring device". |
 |
You may be surprised that there is a blood pressure cuff that uses an iPhone for the digital readout and storage. |
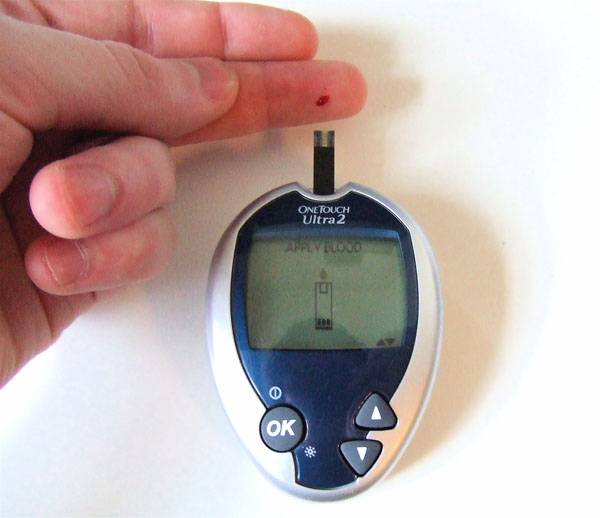 |
MEASURING BLOOD SUGAR: My father is also diabetic and he has had to have is blood glucose measured 3 times a day to determine if he needed an insulin shot.
The science behind these modern blood glucose (sugar) meters is to draw in a set amount of blood. Often it is 1 microliter (a millionth of a liter). The glucose in the blood reacts with an enzyme called glucose oxidase that pulls 2 hydrogen atoms off of the glucose molecule. A third chemical pulls the 2 hydrogen atoms off of the glucose oxidase enzyme. The third chemical then has those 2 hydrogen atoms removed by the use of an electrical current. By measuring how much electrical current it requires to remove these hydrogen atoms, the number of glucose molecules can be calculated. It usually takes about 5 seconds to do that. In the older meters, it took about 2 minutes. So the chemistry behind these meters is quite ingenious.
The readings are usually between 60 and 200. They are measured in mg/dL, which means milligrams of glucose per 1 deciliter of blood. A deciliter is 1/10 of a liter or 100mL. With my father, if the reading is over 200mg/dL, he gets an insulin shot. 200mg/dL is 2,000mg/Liter or 2 grams per liter. 2 grams of sugar is a bottle cap full of sugar. That's quite a bit. |
|
I was surprised to see a home test for A1C because I heard doctors describe it as a specialized test. The A1C test is a common blood test used to diagnose type 1 and type 2 diabetes and to gauge how well a person has been managing his or her diabetes. This test reflects your average blood sugar levels over the last 2 to 3 months. It actually measures the percentage of your hemoglobin that is coated with sugar. They use the term "glycated" to mean coated with sugar. (In chemistry "glyco" is a prefix that refers to glucose or sugars). If this percentage is high, then your body's control of blood sugar is poor and/or you've been eating too much sugar or starches (carbohydrates), which causes your blood cells to be coated with sugar. That sugar doesn't come off of the red blood cells. Blood cells live for 2 or 3 months, which is why this test gives you an idea of the blood sugar level over the last 2 or 3 months.
I couldn't find out exactly how these A1C test strips and meter work, but I imagine it's similar to the way the blood sugar test strips and meter work.
I got the A1C test done last year as part of a check up. My reading of 6.4% is over normal (4% to 6% is normal and over 7% is diabetic). So I cut back on sugar and starches and the reading is now 6.0%, which the high end of normal. Knowing that I can do the test myself with this "AIC Now SELFCHECK AT-HOME A1C SYSTEM", I think I will use the home kit between the 4 months intervals done by the doctor's office. |
|
This is the hemoglobin protein again. Glucose will bond itself to an amino group (-NH2) on the hemoglobin protein. The bond is stable, so glucose stays attached. The percent of the hemoglobin proteins that have glucose attached is the basis for the A1C test. |
|
I also came across test strips that measure glucose and cholesterol at the same time. That sounds useful as well. The science behind it must be like the blood glucose strips and meters, but with an additional enzyme to identify the level of cholesterol. |
|
CHEMISTRY OF URINE TEST STRIPS: There's a lot of chemistry in these multi-test urine test strips. In the chart you can see they test for glucose, pH, protein, ketone, and blood cells. Ketones are compounds where there is a carbon that is double-bonded to oxygen (C=O).
Glucose in urine indicates diabetes. Low pH (acidic urine) can also indicate diabetes. pH strips are often made from pigments from plants such as red cabbage. The pigments change color depending on pH.
Protein in the urine (proteinuria) can indicate a kidney disease or some other serious condition.
Ketones in the urine (ketonuria) indicate the body is having trouble using sugar for energy, so it's using fat and proteins instead. Acetone (CH3-CO-CH3) is one ketone that can be found. Ketones can also be found when a person is on a starvation diet. The test strip has a reagent (a chemical that reacts with some other chemical) that is specific for acetoacetic acid (a common ketone found in urine).
Blood in urine (hematuria) gives people some anxiety but isn't always a reason for concern. Strenuous exercise or too much aspirin can cause it. Infection of kidneys or bladder can also be the cause.
|
|
Urine density can be done using a hydrometer, which uses a floating tube (Left image). The more the inner tube extends above the surface of the urine, the more dense the urine is. The density is read on a scale on the tube. This is the same kind of instrument used to measure the charge of a battery (by measuring sulfuric acid density).
This hydrometer is reading about 1.025. So this urine is 1.025 more dense than water.
|
  |
Another way the density of urine is determined is by measuring how much it bends light (refracts light). This is done with refractometers (2 devices are shown). The computer chip in the devices translate the degrees the light bends into density (specific gravity) of the urine. These devices only need a drop of urine to do the testing. A specific gravity above 1.035 indicates a problem. One problem density can indicate is an imbalance in electrolytes such as potassium and sodium ions.
Hypokalemia is too few potassium ions in the blood. Remember the symbol for potassium is K, which is from the Latin name for potassium, which is "Kalium". So hypokalemia means hypo (too little) potassium (Kalium) in the blood (emia).
Hyponatremia is too few sodium ions in the blood. The Latin word for sodium is "Natrium", which explains why the symbol for sodium is "Na". |
|
PREGNANCY TESTS: Another test that can be done at home is the home pregnancy test. These work by looking for a chemical marker that is associated with pregnancy. One marker is the hormone called human chorionic gonadotropin abbreviated as hCG.
The strips contain anti-hCG globulin, which is an anti-body for hCG. Anti-bodies are proteins created by the immune system to neutralize a foreign bacteria or virus, but also can attach itself to a specific protein. This is a globulin type protein because its shape is globe-like (spherical).
If there is hCG in the urine, the anti-hCG globulin protein will latch onto the hCG protein. There is also a dye attached to the anti-hCG globulin protein. As the urine moves along the absorbent strip, the hCG/anti-hCG/dye group is swept along and forms a pink-rose band. If there is no hCG (person not pregnant), then no band will be seen.
When one band of color gets to the "C", it means the test is "Complete". If there is a band where the "T" is, then it is a positive reading. The "T" means "Test results". In other words, both bands must be seen for it to be a positive reading. Some of these strips don't say "T" or "C" but they show an image that indicates both bands must be present for it to be a positive reading.
|
|
DRUG TESTING: This is another urine test but for the drugs cocaine, amphetamine, THC (marijuana), opiate (morphine/ heroin), and PCP (an hallucinogen known as angel dust on the street). The science behind it is similar to the pregnancy test. There are antibodies for each of these drugs. However, the way you read these urine drug tests is opposite. The "C" means the "Control", but it also signals that the test is complete. So that is similar. However, in the "T" or Test area, a missing line is a positive for the drug. Apparently the drug attaches to the antibody and dye and creates a group that is not carried along by the urine to the T area. Only if the drug is NOT present does the urine carry the antibody and dye combination to the T area. Again, if the antibody-dye combination forms a line at the T area, then that drug was not present. Notice the label in the upper right shows 2 lines as negative and one as positive.
If a line doesn't appear at the "C" /Control area, then something is wrong and the test is invalid.
The 5 strips on the left are dipped into the urine. The urine will then travel along the strip up to the C and T region. When a dye gets to the "C" area, the test is finished, which is the time you look to see if a band is at the T region. |
 |
Another drug test uses saliva instead of urine. The way this one works is similar to the urine drug test. However, 3 drugs are indicated per strip. Saliva is placed on the bottom indentations, and it travels up the strip to where the letters are. "C" is still the control (or complete). COC is for cocaine, mAMP is for methamphetamine, and so forth. Again specific antibodies for each drug is present in the strip along with a dye attached to the antibody. If that antibody/dye group doesn't get to the letters, then the drug is present. |


Yellow is potassium dichromate solution. Dark green is after reacting with alcohol, which forms the dark green chromium(III) sulfate. |
While we are on the topic of abused drugs, I can introduce another drug that sometimes is abused...alcohol. The device is a personal or portable breathalyzer for measuring blood alcohol content in the blood. The legal limit in Arizona is 0.08. They don't normally mention the units, but it's 0.08% w/v, which you should remember %w/v means grams per 100mL. To stay legal, you should have under 0.08 grams of alcohol per 100mL of blood.
This device uses an acid and salt sponge that is sandwiched between 2 platinum electrodes. Alcohol enters this device and passes over this acid/salt/platinum sandwich. The ethyl alcohol (C2H6O) is first converted to acetic acid (C2H5O2). To make that conversion, an oxygen atom is added to the alcohol and a hydrogen atom is removed. The hydrogen atom released is a one proton and one electron. The electron flows down a wire and is measured with an electrical meter which shows up as a reading on the display. The more alcohol present the more electron current will flow, which makes the reading go up. This device is called a fuel cell because it is using alcohol as a fuel to generate electricity. In the future, your cellphones will likely run off of drinking alcohol (ethyl alcohol) or methyl alcohol because alcohols can produce a lot more electricity for a longer period. In other words, your cellphone will stay charged for a month without adding more alcohol to it.
When I worked at the Phoenix Crime lab back in the 70's, the breathalyzer available at that time used a solution of sulfuric acid and a salt called potassium dichromate. K2Cr2O7. Together they also converted ethyl alcohol to acetic acid like the fuel cell here. As the alcohol got converted, the potassium dichromate got converted to chromium(III) sulfate, Cr2(SO4)3. The alcohol was measured by the color change from the yellow potassium dichromate/sulfuric acid solution to a dark green color after the potassium dichromate gets converted to green chromium(III) sulfate. So the Breathalyzer had a light pass through the solution and the change in the color correlated with the amount of alcohol in the breath. To find the amount of alcohol in the blood, the machine multiplied by 2100 because the blood always has 2100 times more alcohol in it compared to the alcohol in the breath. |
|
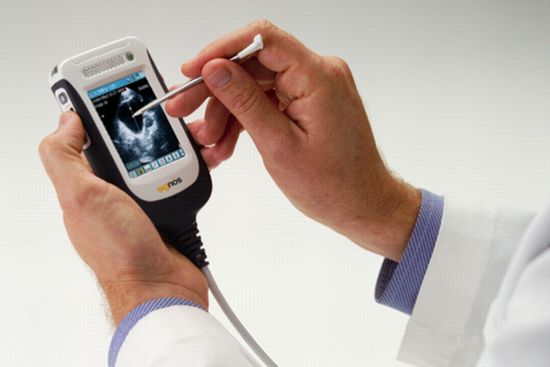
I think the coolest (but not the cheapest) personal medical device is the portable ultrasound imaging machine. Even though it's called the personal ultrasound device, it's marketed more to doctors than the average consumer, but eventually it will be affordable to consumers. |
| The on-campus final will also have some questions that are the same or very similar to the ones on the online final exam given at Sapling Learning. The below practice exam will give some examples of those as well. |
There are three times available for taking the on-campus final in a classroom C329, which is Building C (SE corner of campus) on 3rd floor. Thursday, May 2nd, 7-9pm, Tuesday, May 7th 7-9pm, and Thurs. May 9th 7-9pm. The test will also be in the Testing Center for students who can't make these times; however, you need to make arrangements with their instructor first because the Testing Center has to have your name on a list and have your test on file. If you are in Mr. Costello's class (section 20752), email him at chm130@chemistryland.com to request to take test in the Testing Center.
If you are in Mr. Thacker's class (section 22493), email him at qrt2004@yahoo.com. If you can't come to the campus, contact your instructor for special arrangements. An over-the-phone final may be done instead.
Below is a the practice exam for the CHM130 on-campus final exam.
|
|
What is dangerous about the traditional mercury thermometer?
Answer: It contains toxic mercury and the glass could break.
Are the values on this thermometer in Fahrenheit or Celsius?
Answer: Celsius
|
 |
The left thermometer is reading 37.0°C. What is that in Fahrenheit?
The right thermometer is reading 98.6°F. What is that in Celsius?
Answers:
(37.0°C x 9/5) + 32 = 98.6°F
(98.6°F-32) x 5/9 = 37.0°C
What sensor in the left thermometer is responsible for detecting the temperature and name one of its ingredients?
Answer: A thermistor. (Remember one of the following:) manganese oxide, cobalt oxide, nickel oxide or copper oxide
What sensor in the ear thermometer is responsible for detecting temperature?
Answer: A thermopile (or array of thermocouples).
Name one metal that is used to make a thermocouple.
Answer:
(Remember one of these metals): chromium, nickel, copper, tungsten, or platinum. |
 |
What is the name of this device?
Answer: A pulse oximeter
What is this person's SpO2% reading?
Answer: 98%
What does SpO2% stand for?
Answer: Percent (%) that hemoglobin is saturated (S) with oxygen (O2) in a peripheral (p) part of the body (for example, fingers).
What is this person's pulse (heart rate)?
Answer: 83 beats per minute.
Below what percent should a person get worried?
Answer: Below 90% |
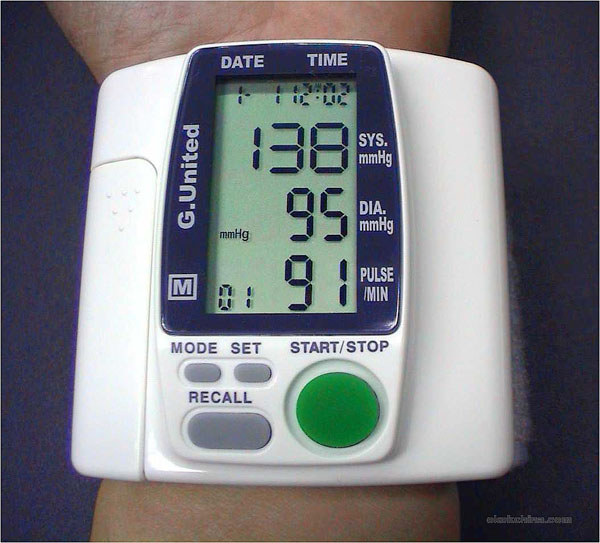 |
Here is a wrist band for taking blood pressure measurements. What is the systolic (high) blood pressure?
Answer: 138 mmHg
What is the diastolic pressure?
Answer: 95 mmHg
What does "mmHg" stand for?
Answer: millimeters of mercury
What is this person's pulse rate?
Answer: 91 beats per minute
Is this blood pressure monitor manual or automatic?
Answer: It's automatic because there's a Start/Stop button
Can this device store blood pressure readings?
Answer: Yes, there is a "Recall" button to recall previous readings. |
 |
What do the numbers 120, 80, and 73 represent?
Answer: 120 is the systolic pressure. 80 is the diastolic pressure. 73 is the pulse rate.
Hypertension is another term for high blood pressure. What systolic and diastolic pressures are considered hypertension? (Note: these are values when a person is at rest).
Answer: 140 and above for systolic pressure. 90 and above for diastolic pressure.
Extra credit: What pressures are considered an emergency situation?
Answer: 180 or more for systolic pressure. 110 or higher for the diastolic pressure.
These blood pressure readings are in millimeters of mercury (mmHg). 760 mmHg is the same as 14.7 pounds per square inch (psi). A pressure of 180 mmHg has how many pounds per square inch on the blood vessels and heart?
Answer:
180mmHg x 14.7psi= 3.5 lbs per square inch.
760mmHg
(So in just a 2 inch by 2 inch section of the heart, the heart is feeling the weight of a bowling ball (14 lbs). You can understand why high blood pressure is something not to ignore.) |
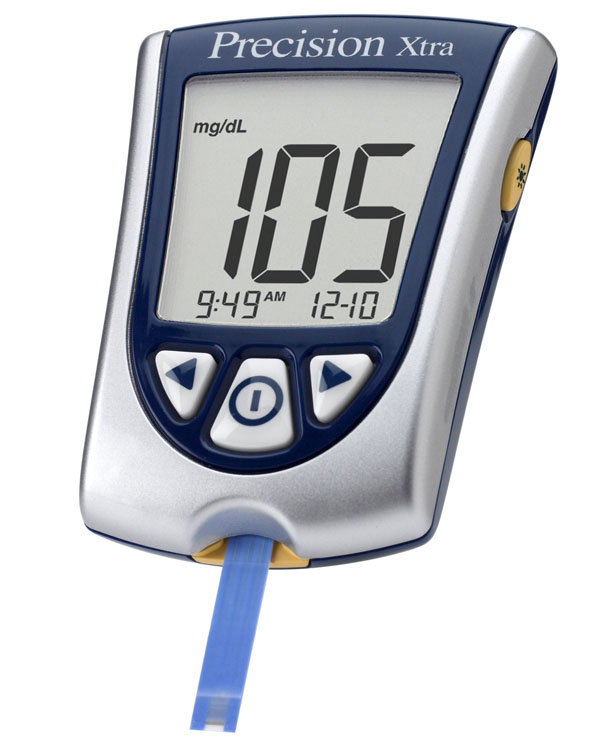 |
To the left is a blood glucose meter and strip. What is the blood sugar level?
Answer: 105 mg/dL
That level is measured in mg/dL. What does mg/dL stand for?
Answer: milligrams per deciliter.
How many milliliters is one deciliter?
Answer: 100 mL
The amount of blood needed to do a test is 1 microliter. If the reading is 105 mg/dL, how many mg of glucose did the glucose meter use?
Answer: 1 microliter means 1 millionth of a liter. Multiply by 1000 for 1000 tests.
1 liter x 105 mg x deci = 0.00105 mg glucose
1,000,000 1 dL 0.1
Notice liter and deci cancel. 0.00105 mg is a very small quantity. These glucose meters are quite sophisticated little machines.
The amount of blood needed to do a test is 1 microliter. If 1000 tests were run, how much blood would be needed?
Answer: 1 microliter means 1 millionth of a liter. Multiply by 1000 for 1000 tests.
1 Liter x 1000= 1 Liter
1,000,000 1000
1/1000 liter is the same as 1 milliliter.
A glucose meter uses the enzyme called glucose oxidase. What does the "ase" mean?
Answer: The "-ase" suffix is usually added to indicate this is an enzyme (a protein that speeds up a chemical reaction).
What does the "oxi" in oxidase indicate?
Answer: "Oxi" usually refers to oxygen. It means that it either adds oxygen atoms or it behaves like oxygen, which is to strip electrons away from things. |
|
What does the A1C test for?
Answer: It determines the average blood sugar level over the last 2 to 3 months. The reading is the percent of the hemoglobin in red blood cells that are coated with sugar (glucose).
The reading in the photo says 6.3 %A1C. What does that mean?
Answer: It means 6.3% of the hemoglobin proteins in the red blood cells are permanently coated with glucose (sugar).
Is the reading of 6.3% high or low?
Answer: It's a little high because 4.0% to 6.0% is normal.
At what reading is a person considered diabetic?
Answer: 7% or higher.
|


|
A person can get urine test strips for home use. They are fairly inexpensive.
The strips in the top image have 10 squares. That means that could test for 10 different substances in the urine.
The below chart is from a urine test strip that measures the concentration of five different substances.
The highest level for glucose says >110 mmol/L. What does mmol/L stand for?
Answer: millimoles per liter
The pH range goes from 5.0 to 8.5. Is pH 5 acidic or alkaline? What about pH 8.5?
Answer: pH 5 is acidic. Anything under 7.0 is acidic. 8.5 would be alkaline urine.
The units for protein in the urine are often written as 1+, 2+, 3+, 4+. 1+ means 30mg/dL. 2+ means 100mg/dL, and 3+ means 300mg/dL. What does mg/dL stand for?
Answer: milligrams per deciliter (or milligrams per 100mL).
If a person has a high reading for ketones, their urine (and breath) is likely to smell like what?
Answer: Acetone (fingernail polish remover)
A high level of glucose in the urine indicates what condition?
Answer: Diabetes
In the terms proteinuria, ketonuria, and hematuria, what does "uria" mean?
Answer: That means in urine or related to urine.
What does hematuria mean?
Answer: It means there is blood in the urine.
|
 |
The home pregnancy test will measure the presence of the hormone called human chorionic gonadotropin. What abbreviation is used for this hormone?
Answer: hCG
Does a colored band at the T region indicate a positive or negative for pregnancy?
Answer: A band at the T region is positive indication of the hormone and therefore indicates the one tested is pregnant.
A band that shows up at the C region means what?
Answer: It means the test is complete and one should then look to see if a band is at the T region.
Hormones, enzymes, and anti-bodies belong to what class of organic compounds?
Answer: They are all proteins, which are chains of amino acids.
Sometimes "globulin" is written after an enzyme name. What does "globulin" mean?
Answer: It means that protein is globular or globe-like in shape. |
 |
This multi-test strip tests for 5 different drugs in the urine. What do the code letters, COC, AMP, THC, OPI, and PCP stand for?
Answer: Cocaine, amphetamine, THC (marijuana), opiates like opium, morphine and heroin, and PCP.
Extra credit. What does THC and PCP stand for?
THC is tetrahydrocannabinol, which is the psychoactive chemical in the cannabis plant (marijuana). PCP is phencyclidine.
PCP comes from letters in the full chemical name, which is phenylcyclohexylpiperidine.
Unlike the pregnancy test, a band of color showing up at the T region on a drug test indicates what?
Answer: A negative. No drug found.
If no color band shows up at the "T" label, what does that mean?
Answer: A positive. The drug is present. Note:
If drug is present, it prevents a band of color from showing up at the T region.
In these kinds of tests, what type of protein is used to identify a particular drug?
Answer: An anti-body that is made for that particular drug. |
 |
This is the saliva version of the urine drug test. This one tests for one extra drug because it has the letters "mAMP". What drug is that referring to?
Answer: Methamphetamine
Amphetamine is an shortened version of the full chemical name. Here is the full chemical name with certain letters highlighted in red. What do those letters spell?
alpha-methylphenethylamine.
Answer: Amphetamine
What is an "amine" group?
Answer: Amines are based on ammonia (NH3) where one or more of the hydrogen atoms are substituted for a carbon atom attached to other atoms. (For example, CH3-NH2 is methylamine.)
The word "methyl" or "meth" indicates a CH3 group. So what do you think is the difference between amphetamine and methamphetamine?
Answer: Methamphetamine is the same as amphetamine but methamphetamine has an extra methyl group on it. |
|
Here is a breathalyzer that uses a fuel cell for the alcohol sensor. Fuel cells use either hydrogen or alcohol to generate electricity. Since people don't breath out hydrogen, then this test uses ethyl alcohol as the fuel to generate electricity. That electricity is displayed on the screen as the blood alcohol content.
The legal limit is 0.08% w/v which is 0.08 grams per 100mL. A person has about 5 liters of blood, so how many grams of alcohol is in their blood?
Answer: 5 liters is 5000mL.
0.08g x 5000mL = 4 grams.
100mL
(That's only about 5mL of pure alcohol, which is the alcohol in 1/3 of a 12 oz can of beer. However, the alcohol in a can of beer will spread out to all water in the body, not just the water in the blood. So it usually takes 3 to 4 cans of beer to reach 0.08 %w/v)
When ethyl alcohol (ethanol) is completely oxidized (burned) in a fuel cell, 12 electrons are produced as electrical current for each molecule of ethanol. The overall final reaction is this:
C2H5OH + O2 → CO2+ H2O
Balance the above reaction.
Answer: C2H5OH +3O2 → 2CO2+3H2O
Each square centimeter of the ethanol fuel cell membrane "sandwich" produces 0.140 watts of power. Your cellphone needs about 1 watt of power.
Instead of the fuel cell in the breathalyzer using ethanol in your breath to send electricity to the display, the electricity could be used to charge your cellphone. . |
If a person has an alcohol level of 0.10 %w/v that means they have 0.10 grams of alcohol per 100 mL of blood. The concentration of alcohol in their breath is always 2100 times less than the concentration in the blood. A person sitting expels about 9 liters per minute. Ethanol has 8 kilowatt-hours of electrical energy per 1 kilogram of ethanol. If all of the alcohol they breathe out is converted to electricity using the fuel cell in the breathalyzer, how many watts of power will the breathalyzer put out? Below is the dimensional analysis of the problem set up in a spreadsheet. You do the math.
| |
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
| 1 |
0.10% w/v |
ratio blood to breath |
Energy content of alcohol |
Respiration rate |
cancel minutes |
cancel m |
|
|
|
| 2 |
0.10 |
g alcohol |
1 |
blood |
8 |
kilowatt-hours |
9 |
liter breath |
60 |
min |
milli |
= |
?? |
watts |
| 3 |
100 |
mL blood |
2100 |
breath |
1 |
kg alcohol |
1 |
minute |
1 |
hour |
0.001 |
|
|
|
Answer: 2 watts (This is great. Intoxicated people could power their cellphones using the alcohol they breathe out.)
Cell K1 says "cancel m". What cell has the milli that column K is canceling?
Answer: B3
The final answer is in watts, not kilowatts. What canceled the kilo in kilowatts?
Answer: (figure out on own).
If the respiration rate goes up, will that produce more or less watts of power?
Answer: (figure out on own).
If the person has a lower blood alcohol level than 0.10%w/v, will that produce more or less watts of power?
Answer: (figure out on own). |
|
The old breathalyzers used a solution of sulfuric acid and potassium dichromate (K2Cr2O7). Below is the reaction without the spectator ions (potassium and sulfate ions).
3CH3CH2OH + 2Cr2O72- + 16H+ → 3CH3COOH + 4Cr3+ + 11H2O
The acetic acid produced has 2 oxygen atoms and ethanol only has one oxygen atom. Where did the extra oxygen atom come from?
Answer: From the dichromate ion
The left side of the equation shows 16 H+ ions. Where did those come from?
Answer: From the sulfuric acid.
Water is produced in this reaction. Here did the 22 hydrogen atoms come from?
Answer: 16 came from the 16 H+ ions. 6 came from the ethanol molecule.
On the exam, you might be asked to balanced one or two of the compounds in the equation, but not the whole equation. |
|
As the alcohol gets converted to acetic acid, the potassium dichromate gets converted to chromium(III) sulfate, Cr2(SO4)3. The alcohol is measured by the color change from the yellow potassium dichromate/sulfuric acid solution to a dark green color of chromium(III) sulfate. So the Breathalyzer had a light pass through the solution and the change in the color correlated to the amount of alcohol in the breath. Below is the same equation as above but showing all ions.
3CH3CH2OH(aq) + 2K2Cr2O7(aq) + 8H2SO4(aq)→ 3CH3COOH(aq) + 2Cr2(SO4)3(aq) + 11H2O(l)
The initial color of the solution is yellow. What is responsible for the yellow color?
Answer: The dichromate ion (Cr2O72-)
After the alcohol is turned into acetic acid, the solution is dark green. What is responsible for that color?
Answer: The chromate(III) ion. (Cr3+)
What is the charge on chromium in the dichromate ion (Cr2O72-)?
Answer: Hint: Oxygen is -2 each. So that's -14 charge. The two chromium ions must reduce a -14 charge to -2 charge. That requires a +12 charge. So what are each of the chromium ions?
|
| The below problems are some pulled from the online final at Sapling Learning. Some of the ones on the actual on-campus final will be variations of the below problems. A few more from the Sapling Learning final will be on the on-campus final, but those are not shown below. |
|
The IV solution is 5% w/v dextrose (5g/100mL). How many grams of dextrose would be in 2.00 liters of 5% Dextrose IV solution? |
|
Let's say you were trapped in a mine and you took 1.2 liters of water and did electrolysis on it to get some oxygen, how many grams of oxygen would you get? (Assume each mL of water weighs 1.0 grams)
2H2O → 2H2 + O2
Answer:
1.2 liters mass |
change g to moles |
ratio from equation |
mole to g O2 |
|
grams O2 |
1200 |
g |
1 |
mol H2O |
1 |
mole O2 |
32 |
g O2 |
= |
? |
g O2 |
|
|
18 |
g. H2O |
2 |
mole H2O |
1 |
mole O2 |
|
|
|
|
|
Water, of course, is H2O.
Which is the most predominant element in water if you base it on its percent of the weight of water?
Which is the most predominant element in water if you base it on its percent of the number of atoms in water? |
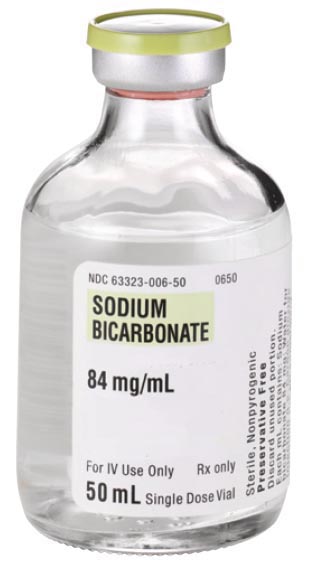
The doctor injects 7.8 cc (7.8 mL) of this solution. How many milligrams of sodium bicarbonate were injected?
Hint: Remember to set up the calculation so the volume of 7.8 mL and concentration of 84mg/mL gives you just mg.
|
|
Teeth begins to dissolve at pH 5. What is the moles per liter of H+ ions at pH 5?
Hint: Two formulas that relate to pH are:
pH= - log (H+ concentration) H+ concentration = 10-pH |
| Orange juice has a pH of about 4. Sipping on it would dissolve your teeth. You want to dilute one liter of orange juice with water so that it would have a pH of 6. That way you could sip on it without dissolving your teeth. To what volume would you need to dilute 1 liter of orange juice to make it a pH of 6?
Hint: pH 4 is 10-4 moles per liter. You want to get it to pH 6. How many times larger is pH 4 compared to pH 6? That is how many times larger volume you need. |
|
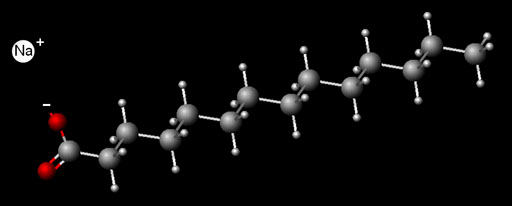
Hard water usually has calcium dissolved in it. The source is often calcium carbonate (CaCO3) or calcium chloride (CaCl2). To the left is a molecule of soap. In water the long chain of soap has a negative one charge (-) and the sodium that was attached has a plus 1 (+) charge. Because of its charge, calcium in hard water grabs onto two soap molecules and causes them to come out of solution to form soap scum. Four ions were mentioned above. What are the charges on the calcium, carbonate, chloride, and sodium ions (in that order)? |
The on-campus exam for CHM130 will be very similar to the practice exam above. |