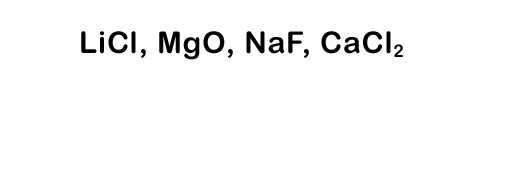Instead of teaching a set of nomenclature rules, which is the normal approach, I'd like to approach your learning of chemistry nomenclature in a way similar to learning a foreign language.
The picture here is of the Louvre Museum in Paris. If you were to move to Paris, you would begin learning the language little by little by listening. In other words you could begin to learn a few words that gives you some idea of what is being said. For example, if you hear "we", you learn that is the French word "Oui" meaning yes. Even if you didn't understand anything else, you would at least know that someone was agreeing with someone else.

In chemistry you don't even need a whole word, but just a syllable to gain some valuable information. For example, you were walking by these two guys and you heard part of the conversation:
"When you mix ___ium ___ide with the ___ide ion the solution turns yellow."
The ending of "ium" tells you that they are probably talking about a metal (like sodium, calcium, potassium, etc.). There are only three exceptions: "helium" and the heavy isotopes of hydrogen: deuterium & tritium.
The syllable of "ide" tells us that this is a compound made up of only two elements. For example, "carbon dioxide." Notice that is made up of just two elements, carbon and oxygen.
The exception is if you hear "___ide ion" That means it's a negative ion of some element. For example, chlorine that has gained an electron to become an ion is called a chloride ion and not a chlorine ion. Positive ions don't use the "ide" ending.
NaCl, CaO, CaCl2, N2O, CO2, KBr, P5O10
Sodium chloride, calcium oxide, calcium chloride, dinitrogen oxide, carbon dioxide, potassium bromide, and pentaphosphorus decaoxide.

I'll show you how valuable this little syllable is.
Let's say you and a friend overhear a conversation. Later the FBI interview both of you saying the people you heard were suspected terrorists. The FBI believe they are shipping a dangerous chemical.
You did hear some chemical names being mentioned. Your friend says he heard "sodium" followed by a word that sounded like "chlorine". You also heard "sodium" but don't remember "chlorine" being said, but you are absolutely sure you heard "ide" at the end of the second word's name.


The word "chlorine" by itself is refering to toxic chlorine gas. In World War I, chlorine gas was released to kill enemy soldiers.
So the words, "sodium, chlorine" could easily mean that the terrorists have both sodium metal and chlorine gas. These are potent and deadly chemicals.


If you have high blood pressure, the doctor is apt to tell you to reduce your intake of sodium chloride (table salt) and instead use potassium chloride.
One of these bottles contain just what you need to prevent a heart attack; unfortunately, instead of the compound being written out as "potassium chloride," it is written as a formula. Both of these elements have potassium and chlorine atoms. Again, "ide" is the clue that the correct bottle is the left one because it has just two elements. The right bottle has 3 elements. It's name is potassium chlorate. If you were to cook with this kind of salt, your food would likely explode. So you see, there's a big difference between ending with "ide" or with "ate".

Learning a language means paying attention to patterns. Let's say below is what you see written:
NaCl, KBr, CaF2
You recognize the elements as sodium, chlorine, potassium, bromine, calcium, and fluorine. Now you hear someone read the compounds outloud as, "sodium chloride, potassium bromide, calcium fluoride." You see a pattern. The first element is read as is, the second element's name is shortened and the suffix "ide" is added.
Here's a list of the element names: lithium, chlorine, magnesium, oxygen, sodium, fluorine, calcium, and chlorine. Try naming the compounds to the left.
To see the answer, roll cursor over the image.
When learning a language, you often encounter things that seem inconsistent. Notice the discrepancy between how these compounds indicate the number of atoms. Some compounds do and some don't. Can you see a pattern here? (roll cursor over image for answer)
Notice that these four compounds of nitrogen are called: nitrogen oxide, dinitrogen oxide, nitrogen dioxide, and dinitrogen trioxide.
At this point, you would conclude that "di" means two and "tri" means three.

When you learn a language, one of the first things you learn is how to count. In chemistry, we use Greek to count.
1= mono (mono isn't used much. 1 is understood)
2 = di
3 = tri
4 = tetra
5 = penta
6 = hexa
7 = hepta
8 = octa
9 = nona
10 = deca

These seven non-metals usually form toxic compounds. So if you ever hear these spoken or see them written, take notice.
Most end with the syllable "ide" and they use the Greek prefix to indicate the number of atoms. For example, the last one says, "phosphorus trichloride". Ammonia could be called "nitrogen trihydride" but the ammonia is so well known, that no one says the full name.
Cl2O7
dichlorine heptaoxide (heptoxide)
N2O4
dinitrogen tetraoxide (tetroxide)
PI3
phosphorus triiodide
We now have some nomenclature rules about naming compounds
that are made of two different non-metals.
1. The first element is written as. If more than one atom of that element,
use the Greek prefix for that number.
2. The name of second element is shortened and the suffix "ide" is added. If more than one atom of the second element, then use the Greek prefix for that number.


When reading chemistry, you see and hear Greek words for counting the elements in non-metal compounds, but when discussing compounds made from a metal and a non-metal, you see Roman Numerals but hear Arabic numerals. It almost makes you want to use your sword on someone.
Written: iron(II) chloride
Spoken: iron two chloride
Written: cobalt(III) oxide
Spoken: cobalt three oxide
CrO2
Spoken: "chromium four oxide"
Written out: Chromium (IV) oxide


Another puzzler: You see the formula CrO2, but are puzzled because someone calls it "chromium four oxide". To you it looks like there's two oxygens not four. The reason for this is that the "four" is referring to the charge on the chromium not the number of oxygen atoms.
Here's a diagram of what chromium and oxygen atoms look
like individually. They start off neutral because the electrons (e-) they
have match the number of protons (p+) they have.
Since each oxygen has a stronger pull on electrons than does chromium,
each will pull off two electrons from the chromium. Chromium has four
outer electrons and these get grabbed by the two oxygen atoms. Now chromium
has 4 more protons than it does electrons, so it is now 4+ charge. The
oxygens have gained 2 electrons each, so they are now 2- charge. Because
these elements now have opposite charges, they stick together to make
chromium (IV) oxide.
Copper(II) chloride > CuCl2
Calcium chloride > CaCl2
Sodium chloride > NaCl
1) Sometimes you hear Roman numerals used and sometimes not.
2) Why did they write two chlorines for calcium chloride (CaCl2), but only one chlorine for sodium chloride (NaCl)?

No need to say more than what's needed:
You wouldn't try to use hand signals to tell your friends here that there's
a Shark-with-a-lot-of-sharp-teeth right behind them. Giving
them the signal for "shark" is all you need.
Chemists are like scuba divers. There's no need to say more than what's necessary. When a group shares a certain amount of knowledge, communication can be shortened because of this shared knowledge.
Chloride ion = Cl-
Sodium ion = Na+ and attracts one Cl-
Calcium ion = Ca2+ and attracts two Cl-
NaCl (no need to say one chlorine because sodium can only attract one)
CaCl2 (no need to say two chlorines, in other words, you would not say calcium dichloride because it's unnecesary when you know that calcium has 2+ charge. That 2+ charge would always attract two chloride ions which have a negative one charge each).
Chemistry nomenclature can be puzzling if we don't have the knowledge that chemists share. What they know is the charge on elements.
Chlorine is a negative one charge, so it's attracted to the positive metal ions like sodium and calcium. When a sodium atom becomes a charged ion, it always becomes 1+ charge. Calcium always becomes a 2+ (two plus) charge.
Copper ion = Cu+ or Cu2+
copper (I) chloride means Cu+ so attracts one Cl- (CuCl)
copper (II) chloride means Cu+ so attracts two Cl- (CuCl2)

MgCl2
Do not say
magnesium dichloride
Saying magnesium chloride is enough

Here's the motto: "Don't tell me something I already know."
It's like saying "yellow bananas", "wet water", or "4 legged horse." Don't use these extra words that are already common knowledge.
On the other hand, don't leave out words that are needed.

The main ingredient of glass is silicon dioxide (SiO2). Sometimes aluminum oxide (Al2O3) is added to make the glass more resistant to chemicals.
Why didn't we say dialuminum trioxide for (Al2O3)? One reason is that we have a metal combined with a non-metal, so we don't use Greek prefixes to count the atoms.
How do we know aluminum oxide has two aluminum atoms and three oxygen atoms?

We refer to this Periodic Table that lists the charges that these elements can have. Aluminum is in the +3 column meaning it loses 3 electrons to become +3 charge. Oxygen is in the -2 column and can have a -2 charge. Having opposite charges, the two are attracted to each other. However, after they are together, they still have an overall +1 charge.
(Al3+O2-)+
That means as a pair, they will attract another negative oxygen atom.
(Al3+O2-)+ ← O2-
This resulting threesome now has a negative one charge (3+ & 4-=1-), so they will now attract a positive aluminum ion.
(O2-Al3+O2-)- ← Al3+
This results in a group that has a plus 2 charge
(Al3+O2-Al3+O2-)2+
Being positive they now attract a O2-.
(Al3+O2-Al3+O2-)2+ ← O2-
Now all together there is no charge, so the compound attracts nothing else and we end with the final compound & formula.
(O2-Al3+O2-Al3+O2-)
which is Al2O3. Also, aluminum never has other charges, so we can depend on it having a +3 charge, so we don't write:
aluminum(III) oxide, we only write aluminum oxide.


Learning the language of chemistry takes time. Like any language, the more you are learn the faster it is to learn more. Also, understanding the logic behind the naming rules helps. Even though there is quite a bit of memorization in nomenclature, remember, it's not just names that are important, it's the chemical behind the name.
The payoff with learning how to speak chemistry is that it lets you communicate with so many others around the world that also have learned the language of chemistry.