
Chaos:
In an earlier tutorial I talked about our need to combat chaos by ways to simplify and classify the world around us. That tutorial was focused on the development of the Periodic Table of the Elements, which allowed us to classify all of elements in the cosmos, which reduced the feeling of chaos.
Elements are made up of atoms. So we can't really separate the understanding of elements and the understanding of atoms. So part of this tutorial will overlap with the tutorial on how elements were classified with the Periodic Table.

Four Elements:
Around 440 B.C. Empedocles suggested that there were four elements: Water, Earth, Air, and Fire. It seemed logical because when things caught fire, moisture is released, air can be felt coming up from it, and the ashes show the earth that it contained. Classifying all matter as only being made of four elements certainly simplified our view of the world.




Democritus was quite perceptive. In a manner similar to atoms, he proposed that the Milky Way was not milk nor clouds but a collection of millions of stars that individually are too small to see but all together they look like a cloud or liquid.
It is quite amazing that he saw this pattern at both the large scale of a galaxy and the small scale of atoms. The pattern is that we think we see something smooth, but that's only because of the limitaton of our vision or touch. On closer inspection, there's always individual pieces, not something continuous.



Around 340 B.C. Aristotle said he didn't believe in the theory of Atoms because you would be putting a restriction on the gods. If the gods wanted to divide an element to something smaller than an atom, they could. The concept that God or gods had unlimited power was quite popular and it kept people from accepting the idea of atoms (something indivisible) for about 2,000 years.
In addition Aristotle introduced the fifth element that he said all heavenly bodies (Sun, moon, and stars, etc.) were made of. He also said the fifth element could turn cheap metals into gold and cure disease and old age. This started alchemy, the pursuit of the 5th element.

Born in in Ireland in 1627 was Robert Boyle. He wrote a book called the "Skeptical Chemist." Now available on Amazon.com. Actually, a book about him and his book is available on Amazon.com.
His book urged chemists to abandon the view that elements are mystical substances. In other words he criticized Aristotle's supernatural fifth element and alchemy. Instead he promoted a philosophy that valued observation and experimentation.

ELEMENTS: Boyle also said the term, element, needs a precise meaning. An element is a substance not capable of being broken down or decomposed into simpler substances.
Earth can’t be an element because it consists of
simpler substances like gold, mercury, iron, and so forth. These are elements
because they can’t be broken down to something simpler. Later French
scientist Lavoisier would reinforce that notion.

AIR SUGGESTS PARTICLES (atoms): Boyle is most known for his work with gases. With the help of inventor Robert Hooke, he built an air pump. This allowed him to discover many things about the properties of air.
He discovered that air was required for combustion, respiration, and for sound.
Working with gases made him come to a conclusion about
the nature of matter (especially gases).

GAS IS ATOMS: Because of the nature of gases, he believed in the corpuscular theory of atoms agreeing with Democritus and disagreeing with Aristotle.
Corpuscular means that air is made up of particles. Others may have thought air to be more like a sponge or compressible liquid, but he thought air was made up of individual particles like atoms. These particles turned out to be a pair of nitrogen atoms and a pair oxygen atoms. Plus, some particles were single atoms of argon, neon, and krypton in the air.


These are the actual wood spheres that Dalton used as models for atoms. They are about 200 years old. Notice the holes drilled in them. I imagine he used them to connect atoms to other atoms to make compounds.
His models are actually very similar to the ones we buy now to model atoms and molecules. Yes, 200 years of technological advancements and our models are not any better.

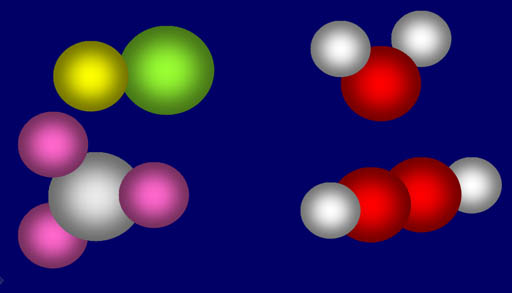
His next idea would tremendously simplify the complexity of all the materials around us. Dalton suggested that substances around us were made up from a grouping of specific number of atoms of different elements. For example, a water molecule is made from two atoms of hydrogen and one atom of oxygen (upper right). If there's 2 oxygen atoms and 2 hydrogen atoms (lower right) then it's not water but something else (hydrogen peroxide). Salt is made from one sodium atom and one chlorine atom. Ammonia is made from 3 hydrogen atoms and one nitrogen atoms (lower left).


So Democritus, Boyle, and Dalton visualized atoms as particles. Democritus thought they had different shapes, but Dalton thought they were spherical. Dalton also thought the atoms of a particular element would all be the same size and weight. Atoms of other elements would have a different size and weight. This way of visualizing atoms is still popular 200 years later, and it's the way they are often drawn.

Born in 1776 in Italy Amadeo Avogadro was a public administrator, but developed an interest in science. After hearing a lecture from Joseph Gay-Lussac regarding gases, Avogadro decided to also study gases. He was impressed with Gay-Lussac’s data collection methods which involved trips in balloons which rose 4.5 miles above sea level!

Example: 2 liters hydrogen combine with 1 liter oxygen to yield 2 liters water vapor. These simple whole number combinations hinted that gases are particles (atoms) that distribute themselves evenly.
Avogadro reasoned that if gases react in small whole numbers, then all elements in gas form must distribute themselves equally and according to number. In other words, one liter of hydrogen has the same number of molecules as a liter of oxygen. Measuring gas volume provides a way to indirectly count atoms, which means you can determine what proportions elements combine to form molecules. This gives you its formula. A pretty good trick when you think that everything is invisible.



In 1897 Sir John Joseph Thomson discovered that electrons can be stripped off of metal atoms. He did this by using a glass tube that had most of the air removed. In the tube were two metal plates. One had a high negative charge and the other a high positive charge.
Thomson discovered that the "beam could be bent by magnetism or electrical charge. He also measured that the particles in the beam were a couple of thousand times lighter than the hydrogen atom. He concluded the particles had been stripped off atoms. This was the discovery of electrons and the realization that the atom (meaning indivisible) had actually been divided because the electrons of the atom had been stripped away.

Plum Pudding Model:
Thomson proposed that the atom had these negatively charged corpuscles (later called electrons) that floated in a cloud of positive charge. So the electrons were like plums baked in a bowl of pudding. The positive charges (protons) filled the whole atom (pudding).


