SYMBOLS VERSUS THE REAL
THING

What fraction is this?
If you said "ten one-hundredths," then you are
technically not correct.
This is not a fraction. The proper name is called a fractional numeral.
Textbooks and teachers get lazy and usually call them fractions.
Roll cursor over the image to see the real ten one-hundredths.

What went wrong?:
Somewhere in math education, the symbols for mathematics got confused
with being mathematics.
Mathematics describes actual quantities. Unfortunately, math training tends to focus more on the symbols than the actual quantities.

Get Priorities Straight: When we focus too much at symbols, we begin to believe that the symbols are more important than the real thing.
Here's a question for you. Would you rather get the grade of "A" for math but not really learn it or get a "B" and actually have a mastery of math?
In other words, we need to focus on what these symbols represent, not the symbols themselves.

Symbols of Time:
It's interesting that we can hardly think of time without thinking of the symbols for time. When we communicate time, we almost alway use these symbols. Before clocks were so abundant, people would normally base time on the sun, such as, day break, high-noon, and sundown.
Emotions as Punctuation?
With the advent of online chatting and email, it seems that keyboard
symbols are now used to represent emotions.
When you see a smile symbol ":)", is the person really smiling or do they want you to think they are smiling? Again, it's not the same as smiling.

What's in a Name?
Symbols have gotten so valued that a person put a tattoo of his family name on his arm. We just hope he cares about his real family as much as he does the family name.

It's All in the Mind:
Symbolism is so strong that burning a symbol is equated to harming what the symbol represents.
It's like writing down the word "taxes" on a piece of paper and then burning that piece of paper. No, you still have to pay taxes. Yes, we can tell you don't like taxes.

Watch Out for Symbols!
E=mc2 is a formula made of symbols for energy, mass and the speed of light, but the image better shows the real E=mc2.
There's a big difference between the symbol and what it stands for.
It would be an interesting world if the symbols had the same power as what they represent. Very few people would dare write E=mc2.

You Aren't Fooling Us:
Lifting the symbol for 500 pounds isn't that impressive. Again there's always a big difference between the symbol and what it represents.
Symbol Quiz:
Try to answer the questions on the left. To see the answer roll cursor over the question.
Answering these questions will help you separate the symbol from the thing it represents.
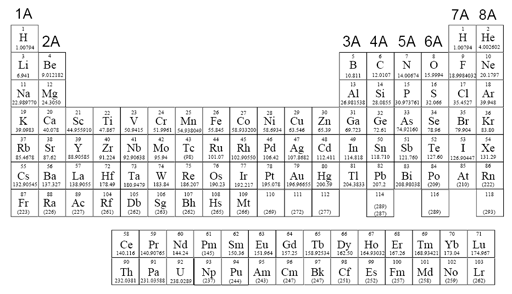
Find a Better Name:
Here is the Periodic Table of the Elements. What is a better name for this table?
Roll cursor over title to see the better name.
The problem with the symbols of the elements is that it makes all elements look the same. For example the letters make them all look the same size and the same color. Notice Cu, Ag, and Au are copper, silver, and gold respectively, but the symbols give us no clue to what they look like. Focusing just on symbols for elements is a lousy way to learn about the elements.
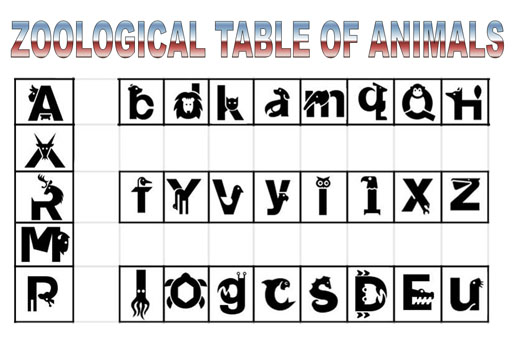
Paper Tiger:
For example, let's say you are taking zoology and they present you with
a table with symbols for animals. The symbols make all animals look the
same size and color. You miss out on the real look and behavior of animals.
You would soon grow bored with learning about animals because symbols
are a poor substitute for studying the real thing.
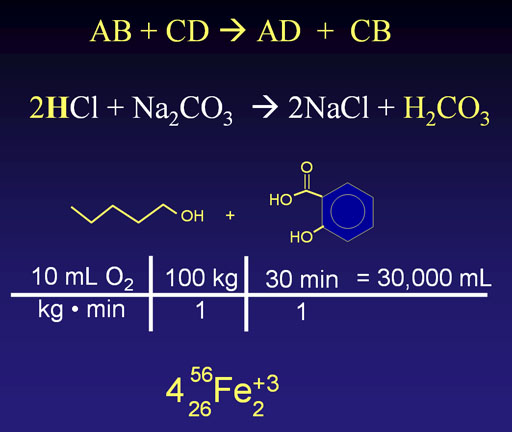
Chemistry uses symbols heavily. Some symbols are for elements, some are math symbols, some are measurement symbols, and some are symbolic lines. Many symbols are confusing. Sometimes the identical symbol can have various meanings depending on context. For example, the top line include the letters "B" and "C" which if element symbols, would mean boron and carbon; here they means something else. Likewise, the plus "+" symbol can have many different meanings.

Legal Torture: If you only think of chemistry as mostly symbols, I guarantee confusion, boredom, and a feeling of being tortured.
Yes, chemistry uses a lot of symbols, but chemistry is not symbols. Chemistry is a hands-on science, which means you can see, touch, smell, and feel it. Symbols are fine, just learn what they stand for, which is something physical and tangible.

Imagine whatever you touched, it turned into the piece of paper with the symbol of that object. For example, you try to take a shower and the water would become pieces of paper. Some saying "water" and others saying "H2O". Now let's say you tried to pet your dog. At first the fur on the dog would converted to pieces of paper. Some saying "protein"; some saying "keratin". Others saying "fur". Then the dog disappears leaving pieces of paper with "dog, canine, hound, perro, hund, etc.". Imagine a world that is reduced to symbols. It would be a flat and monotone. Imagine a subject reduced to symbols. It's almost as bad.

Imagine whatever chemical word or symbol you wrote, it would magically become the real thing. For example, if you wrote "gold" the letters would turn to gold. If you wrote "10 pounds of gold", the paper would get real heavy as a 10 pound block of gold appeared. If you wrote "nitroglycerin" or "Leopard", you would have be careful. Likewise, if you wrote "sodium" or "Na" the letters would turn silvery and start to burn the paper. Hurry and write "chloride" or "Cl" and the sodium turns into table salt (NaCl). Imagine chemistry where all you had to do was write the symbol and that substance would appear. That would be a fun way to learn chemistry. So never write a symbol without imagining it coming into existence right in front of you. If you do that, you will learn the subject quite well.
Mathematical and Chemical Symbols
.

Chemistry uses a raised dot "·" to represent attachment of water molecules. Here is the salt that makes up Epsom Salts. It's called magnesium sulfate heptahydrate.
MgSO4·7H2O. The raised dot indicates that the water molecules are bonded to the salt.
Dots are also used to represent electrons. In "Na·" the dot is used to represent the single, outer electron that sodium (Na) possesses. You will learn more about this in Chapter 11.
Dots are also used in chemistry to represent an unshared (unpaired) electron which normally is shared. For example, chlorine atoms go around in pairs, represented as Cl2 or Cl-Cl. Each chlorine atom is sharing one of its electrons. However, if the bond is broken and the electrons are no being shared (no longer paired) we get two "Cl·". This is called an chlorine radical which is even more reactive and dangerous than chlorine gas (Cl2). So even though a small dot doesn't look like much, it can mean the difference between unhealthy and deadly.
Here's another example. Milk of Magnesia, Mg(OH)2, is used to neutralize excess acids in the stomach. The hydroxide ion, OH-, is what neutralizes the excess acid (H+) in the stomach. OH- + H --> H2O. However, if you had that much OH· in your stomach, that would kill you. OH· is called the hydroxyl radical and will easily chemically react with tissue in the stomach. So the difference between OH- and OH· is the difference between medicinal and poisonous.
-
-
—
—
The dash "-" would be the second most simply written symbol. The dash also has different meanings. In math it means both subtraction and negative. In this equation, "-2 - 5 = -7" The middle dash means subtraction (take away) and the other two mean that the numbers are negative numbers. "-" is called a subtraction sign or a negative sign. They don't mean the same thing. A longer dash is used for the fraction bar, also called the "vinculum".
3
4
In English, the dash is a called a hyphen and is used to hyphenate words like "under-prepared" or Mary Smith-Wilson.
In chemistry the dash is used to represent a single bond (sharing of one electron each) between two atoms. For example, in Cl-Cl the dash is showing a bond between two chlorine atoms. If that bond is broken using UV light, we get Cl· + Cl·. Remember the dot represents an electron that normally was being shared (paired) but now is unpaired.
In chemistry a superscript dash is used to indicate a negative charge. For example, Cl- means a chlorine atom with an extra electron. It's called the chloride ion. So notice the difference between Cl-, Cl-, and Cl·.
+
+
+
+
Another simple symbol is the plus sign "+". In math it can mean "add" or "positive". In this example, "(-3) + (+4)= 1" the first "+" means to add. The second "+" means that 4 is a positive 4.
In electricity and chemistry, the "+" and "-" symbols are used to represent an electrical charge. For batteries, the positive "+" terminal is where there is a deficit in electrons. The negative "-" terminal is there there is an excess of electrons. Chemistry is similar. Mg2+ means the positive protons in magnesium out number the negative electrons by 2 giving it a positive 2 charge. In other words, the magnesium atom lost 2 of its electrons. S2- means that sulfur has gained two extra electrons and now has a negative 2 charge.
In chemistry equations, the "+" means compounds are mixed with each other (i.e., exposed to each other). For example, in 2H2 + O2 --> 2H2O the "+" means hydrogen is mixed with oxygen. With heat or a spark, they combine to form water.
In chemistry, the "+" sign can also mean a compound rotates polarized light in in a clockwise direction. So "+-glucose", is a type of glucose that rotates polarized light in a clockwise direction. Note, the dash "-" after the "+" is an English hyphen and not a math "subtraction" sign or a math "negative" sign. Don't you just love symbols.
. - +

Symbols are great shortcuts but they also make a subject difficult to learn. Focusing too much on symbols will give you headaches, sore eyes, and a feeling of being weighed down. You must balance symbols with pictures, models, or hands-on learning. For example, all the boring formulas and symbols you see in this picture actually represent something interesting or important. For example, in the lower right we see H2O2. That is hydrogen peroxide. If you do a Google Image search for H2O2, you will find some interesting pictures. See the next panel.

The first image to catch my eye was the jet pack. It is powered solely by H2O2. Below the jet pack is a rocket. It used H2O2 as a source of oxygen to burn kerosene to make a powerful rocket fuel. In the upper right, I found this picture with an article about an affliction that Michael Jackson supposedly has called vitiligo. According to the article, vitiligo causes excess H2O2 to be produced in the skin which bleaches out the dark melanin pigment in skin. They also said that Michael chose to bleach all of his skin so it was uniformly white.
The right middle picture was a woman who used hydrogen peroxide to bleach her hair. It took several days. This is where we hear the phrase "peroxide blonde".
The bottom right picture is a blood "milkshake" made with cows blood and hydrogen peroxide. An enzyme in the blood called "catalase" decomposes the hydrogen peroxide into water and oxygen. The oxygen forms the bubbles. The enzyme is so efficient that just one of these catalase molecules can decompose 6 million hydrogen peroxide molecules every second.
Yes, the symbol for hydrogen peroxide "H2O2" is quite boring, but when you see what it really represents, it's a lot more interesting. A follow up to looking at pictures is to buy some hydrogen peroxide and learn how it smells and even tastes (dilute it and taste it). Pour it on the next cut you get and you will see that amazing enzyme, catalase, turn the hydrogen peroxide into water and oxygen.








