
Because the chemistry kit for this distance learning version of the CHM130LL is portable, it means it can be geared towards field chemistry (chemistry done in the field or away from a standard laboratory). Below are some areas of discipline that utilize field chemistry.
The kit on the left is for CHM107. The CHM130 kit is similar but with a more rugged case.

Hydrology: Study of water sources, drainage, and water content in soil and air.
Environmental chemistry: Study of contamination in water, soil, and air
Geochemistry: Identification of minerals and their physical and chemical changes.
Mining: Identification of ores.
Forensics: Identification of physical evidence at crime scenes
Haz Mat Response: Identification of hazardous materials and their neutralization and cleanup.
Agriculture and Forestry: Analysis of soils.
Hydroponics: Creating nutrient solutions and monitoring of pH, nutrient levels, and light levels for growing plants.
Archaeological Excavations: Identification of artifacts and their immediate surroundings.
Healthcare Outreach Programs: In addition to medical treatment, some medical lab tests are done in the field.
Experiment 1: Measurements (Measurement of Length, Volume, and Mass was on-campus title)
Objectives
1. Use measuring tools correctly (read and record measurements correctly and with proper number of significant figures)
2. Become familiar with Metric units of measurement.
3. Become familiar with the measuring tools in your kit.
4. Know how to use factor-label conversion methods to convert the units of measurement and to solve problems based on dimensions.
(This is from on-campus lab 2)5. Apply these skills and knowledge
Theory and Applications (To be developed)
Procedures (To be developed)
Post-Lab questions and assignments (To be developed)

Experiment 2: Determining the Total Dissolved Solids (TDS) in a Solution
(The on-campus lab 2 has a section called "Percent Composition" where students find the weight percent of salt in a salt solution. TDS is similar to that but is a term they may hear in various applications. So this lab focuses on how to measure TDS.)
Objectives
1. Learn the meaning and uses for finding the TDS of a sample.
2. Determine the TDS value for a water sample from a local body of water and from your home using both of the below methods.
2a. Find TDS using a gravimetric method.
2b. Find TDS using a conductivity method.
Theory and Applications (To be developed)
Procedures (To be developed)
Post-Lab questions and assignments (To be developed)


Experiment 3: Density
Objectives
1. Learn the definition of density and its usefulness.
2. Measure the volume of objects using geometry, water displacement, and buoyancy methods.
3. Determine the density of a solid and a liquid using mass/volume. Also, determine density by making a graph of volume versus mass and then finding the slope.
Theory and Applications (To be developed)
Application ideas
1. Find density of an unknown mineral to help identify it. This would be useful in geology and mineralogy.
2. Find density of a metal object found at an archeologicial site to help identify the metal and confirm its authenticity.
3. Find density of unknown liquid that was dumped in the desert to help identify it. (perhaps use mineral oil or glycerin as the unknown liquids).
Procedures (To be developed)
Post-Lab questions and assignments (To be developed)


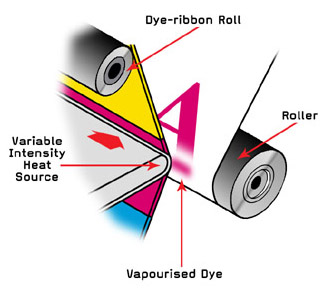
Experiment 4: Chemical and Physical Changes
Objectives
1. List physical and chemical properties of substances and explain how they are used in identification and separation of substances.
2. Outline the distinguishing characteristics of physical and chemical change.
3. Distinguish between physical and chemical processes.
Theory and Applications (To be developed)
Application ideas
1. In geology the test for carbonate minerals is done by looking for bubbles when a small amount of weak acid (like 0.1M HCl) is dropped onto the rock. That will be a chemical change.
2. In mining and geology the identification of ores is important. This web page has more information:
http://cavemanchemistry.com/oldcave/projects/smelt/
3. In forensics or Haz Mat situations where you are trying to identify an unknown white powder, looking for bubbles when a weak acid is added helps to identify everyday carbonates such as baking soda, washing soda, and chalk. Also, heating a sample and noticing its behavior helps in identification. Solubility in water is another way to categorize these unknowns.
4. More ideas of physical and chemical changes may be found in the below webpage:
http://en.wikipedia.org/wiki/Alchemical_symbol#12_core_alchemical_processes5. Dye-sublimation printing is based on transfer of dye to paper using sublimation (a physical change). Sublimation of these kind of inks (dyes) may be useful for a lab exercise here. Also, some stain removing procedures seem to take advantage of sublimation of the dye that caused the stain.
6. Instead of electrolysis of water in lab 5, it could go here or go on the Oxygen lab (Lab 7).
Procedures (To be developed)
Post-Lab questions and assignments (To be developed)

Experiment 5: Separation of Mixtures
Objectives
1. Apply techniques (filtration, decomposition, solubility, evaporation, magnetism, etc.) to separate the components of a mxiture.
2. Review physical and chemical properties.
Theory and Applications (To be developed)
Application ideas
1. Gold dust is often found in a mixture of black sand (mostly magnetite), regular quartz sand, calcium carbonate, and some water soluble minerals. The task for this lab is to isolate the gold dust from the mixture.
Procedures (To be developed)
Post-Lab questions and assignments (To be developed)
Experiment 6: Nomenclature (This "Lab" is used in the on-campus lab, but it's not really a lab. It a competency) This lab could be turned into a lab for Inorganic Analysis.
Objectives (these are the on-campus objectives. These would be moved to chemistry concepts)
1. Differentiate between binary and ternary compounds.
2. Learn names and correct charges of polyatomic ions.
3. Write names and formulas for compounds.
Theory and Applications (To be developed)
Application ideas
1. Have small samples of many of the compounds on page of 48 of the lab manual. Students can do the same activity of determining which ion is responsible for the color of the compound.
This link may be useful:
http://en.wikipedia.org/wiki/Qualitative_inorganic_analysis
2. Use nitrate/nitrite test paper to test for nitrates and nitrites in your home water and in the sample from local irrigation water source (if nitrate fertilizer is in water, it will be detected).
3. Use pH paper and phenolphthalein to test for hydroxide ion (OH-).
Procedures (To be developed)
Post-Lab questions and assignments (To be developed)
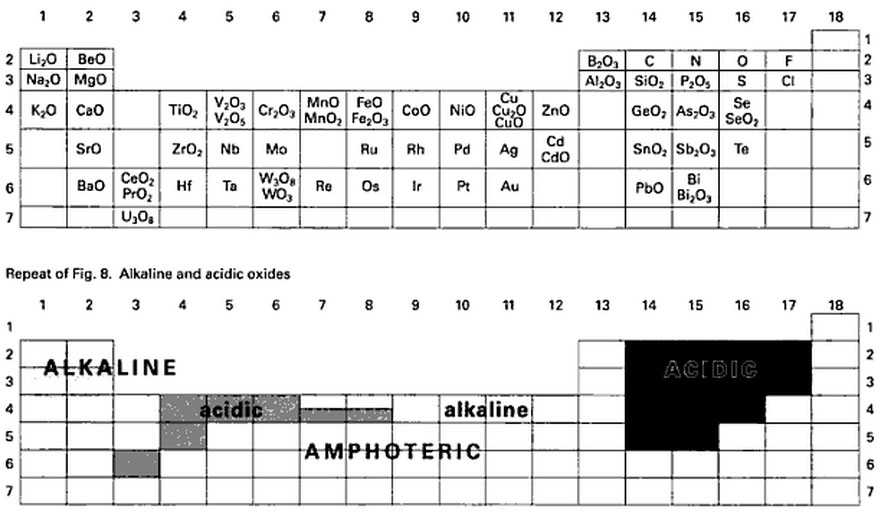
Experiment 7: Oxygen - Preparation of Oxygen and Behavior of Oxides.
Objectives
1. Prepare oxygen by a decomposition reaction.
2. Observe and record some chemical and physical properties of oxygen.
3. Dissolve some oxides in water and check whether acids or bases were formed.
Theory and Applications (To be developed)
Application ideas
1. Instead of potassium chlorate, the online students can use 3% hydrogen peroxide to generate oxygen. Some of the activities done in on-campus lab with oxygen can be the same.
2. Calcium and magnesium oxides are components of portland cement. Checking the pH of a small sample of portland cement after mixed with water will show how metal oxides react with water. This activity also shows how one can identify cement.
Properties of s- and p-Block Elements Li Be B C N O F Na Mg Al Si P S Cl K Ca Ga Ge As Se Br Rb Sr In Sn Sb Te I Cs Ba Tl Pb Bi Po At Basic Oxides Amphoteric Oxides Acidic Oxides
3. The burning of sulfur done in the on-campus lab can also be done with smaller amounts. It has relevance to enviromental chemistry.
Procedures (To be developed)
Post-Lab questions and assignments (To be developed)
Experiment 8: Single Replacement (displacement) Reactions
Objectives
1. React metals with water or dilute acid to produce hydrogen gas.
2. List metals in order of activity based on your observation.
3. Use the activity series to predict ability of metals to produce hydrogen from water or acids.
4. Write balanced chemical equations for single replacement reactions.
Theory and Applications (To be developed)
Application ideas
1. Sacrificial metals on hot water heaters, boats, bridges, and other structures can give some application of single replacement reactions. Galvanized steel, which has a zinc coating, is another example of a sacrificial metal.2. Creating a make-shift battery would be an application of single replacement reaction. The kit will have a multimeter which will indicate the voltage of these batteries.
3. (Forensics) Copper theft is a rampant crime now since the price of copper is so high. Copper in contact with another metal (like iron or aluminum) is likely to create some corrosion due to single replacement reaction (galvanic corrosion). Evidence of this corrosion could show that a suspect had copper tubing or wire in contact with their steel or aluminum tool box. A student can recreate this corrosion using aluminum and copper.
4, The kit will have magnesium strips. So that could be used for single replacement reaction.
Procedures (To be developed)
Post-Lab questions and assignments (To be developed)
Experiment 9: Double Replacement (displacement) Reactions
Objectives
1. Review general pattern for double-replacement reactions.
2. Predict whether a reaction will occur based on a few simple rules.
Theory and Applications (To be developed)
Application ideas
1. (Haz Mat). Neutralization of acids and bases involves a double-replacement reaction. Sodium bicarbonate could be used for both.2. (Haz Mat/Forensics) A double replacement reaction between silver nitrate and a halide is used for identification of halides. Some drugs are made into a hydrochloride salt to make them more water soluble. The identification of chloride helps determine whether it is the "free base" (unprotonated amine) version or the hydrochloride version.
3. (Agriculture/Hydroponics) Ammonium nitrate is used to provide nitrogen for plants. If the pH is too high (alkaline), then a double-replacement reaction occurs forming ammonium hydroxide. Ammonium hydroxide then decomposes into ammonia and water.
4. Ca(NO3)2 is used in hydroponics to provide a source of calcium and nitrate. Calcium ions will form a precipitate with oxalate ions (a source could be Na2C2O4). This would be a double-replacement reaction to test for calcium; however, the toxicity of sodium oxalate may be too high to have in a kit, but this could be an example.
5. (Forensics). Silver nitrate solution is used to develop fingerprints. It reacts with the chloride from NaCl in the sweat. 1 gram silver nitrate in 100mL of water (1% w/v).
Procedures (To be developed)
Post-Lab questions and assignments (To be developed)
Experiment 10: Properties of Ionic and Covalent Compounds
Objectives
1. Describe the characteristics of ionic and covalent compounds, and compare some of their physical and chemical properties.
2. Extract a material from one solvent to another.
3. Study some properties of soaps and detergents.
Theory and Applications (To be developed)
Application ideas
1. (Haz Mat/Forensics). Heating of a sample and noting its behaviour helps categorize a compound as ionic or covalent. This helps in the identification of an unknown powder.2. The cleaning of raw biodiesel takes advantage of the fact that the contaminants in the raw biodiesel are polar (or ionic) and that biodiesel is non-polar. So water can be used to dissolve the polar contaminants, plus water will separate from the biodiesel. If too much NaOH is added during the reaction of methanol, NaOH, and oil, then soap will form causing an emulsion of the biodiesel and water.
3. The titration of fatty acids in used cooking oil prior to converting it to biodiesel requires isopropyl alcohol to dissolve the oil (and fatty acids) but also allows it to be partially soluble in the sodium hydroxide titrant. This allows the OH- to get to the acidic hydrogens on the fatty acids. So it's a good lesson in ionic and covalent substances and their solubility.
3. Removing stains with a non-polar solvent is similar to the iodine extraction activity in the on-campus lab. Instead of kerosene, we could probably use biodiesel or vegetable oil. Instead of iodine, we could probably use a food coloring dye. I will see if some of the food coloring dyes can be extracted from an aqueous solution to a non-polar solvent.
Procedures (To be developed)
Post-Lab questions and assignments (To be developed)
Experiment 11: Properties of Solutions
Objectives
1. Classify compounds according to their behavior in dilute water solution.
2. Observe heat of solution.
3. Prepare and use a solubility curve.
Theory and Applications (To be developed)
Application ideas
1. (Haz Mat/Forensics/beverage testing). Checking the conductivity of a solution gives clues to the identity of the chemicals in a solution. Measuring the ohms resistance in a solution using the kit's multimeter will give a semi-quantitative analysis of the level of electrolytes in the solution. (Test samples could include Pedialyte and some popular electrolyte sports drinks.)
2. (Haz Mat/Forensics/First aid). When a solid is dissolved in water it may release or absorb heat. This information is useful for aiding in identification of an unknown powder. In cases where the identity is known or not important, the heating or cooling affect of the powder dissolving is useful for first aid.
3. (Haz Mat/Forensics/Environmental Chemistry). Solubility of a compound can be used to help identify a compound. Environmental chemistry and Haz Mat operations will use the data to see how much will dissolved by water (rain or fire hoses) and get into lakes, rivers, or ground water.
Procedures (To be developed)
Post-Lab questions and assignments (To be developed)
Experiment 12: Introduction to pH, Household Products, and Buffers
Objectives
1. Check the pH of aqueous solutions and household products using various indicators and a pH meter.
2. Identify the buffer solutions.
Theory and Applications (To be developed)
Application ideas
1. (Haz Mat/Forensics). Checking the pH of a solution gives clues to the identity of the chemicals in a solution. The field kit can use the following indicators: cabbage extract, methyl red, phenolphthalein, bromthymol blue, 0-14 pH papers, and blue/red litmus papers.
2. (Haz Mat/First aid). Even though a strong base can neutralize an strong acid spill, using too much base will create an equally dangerous situation. A buffer solution is the preferred way to neutralize a strong acid or strong base. The student will see how a buffer works then make up a buffer. First aid treatment of insect stings also use a buffer solution to neutralize formic acid.
Procedures (To be developed)
Post-Lab questions and assignments (To be developed)